Patients with severe COVID-19, such as individuals in intensive care units (ICU), are exceptionally susceptible to bacterial and fungal infections. The most prevalent fungal infections are aspergillosis and candidemia. Nonetheless, other fungal species (for instance, Histoplasma spp., Rhizopus spp., Mucor spp., Cryptococcus spp.) have recently been increasingly linked to opportunistic fungal diseases in COVID-19 patients. These fungal co-infections are described with rising incidence, severe illness, and death that is associated with host immune response. Awareness of the high risks of the occurrence of fungal co-infections is crucial to downgrade any arrear in diagnosis and treatment to support the prevention of severe illness and death directly related to these infections.
- fungal infection
- COVID-19
- SARS-CoV-2
- Candida
- Aspergillus
- Mucor
- immune response
- microbiome
1. Introduction
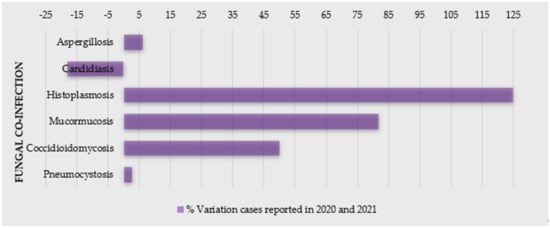
2. Fungal Infections as a Co-Morbidity of COVID-19
Fungal co-infections are frequent in the COVID-19 patients; therefore, its awareness is important for proper diagnosis and, subsequently, efficient treatment of the fungal co-infections for reducing morbidity and mortality. Due to a general neglected approach towards fungal tropical diseases, morbidity and mortality is expected to worsen in the context of the COVID-19 pandemic [22]. SARS related to COVID-19 disease is known to increase the risk of invasive fungal infections (IFI) [23][24][23,24]. In addition, patients suffering from endemic mycoses and COVID-19 co-infection seem to be an at-risk population and have a poor prognosis. A significant number of cases of COVID-19-associated candidiasis, aspergillosis, mucormycosis, and histoplasmosis have been reported so far from the different region of the world [22][25][26][27][22,25,26,27]. Some reports even state that COVID-19 increases the mortality rate in the patients having fungal infections, but the case reports suggest that individuals with COVID-19 are more susceptible to a fungal infection mostly because of impaired immune responses, which further increases the awareness of clinicians for more effective diagnosis and treatment [28][29][28,29].2.1. Candidiasis
| Fungal Infection in COVID-19 Infection | Observed Immune Response |
Co-Morbidity/ Disease Models |
Test/Diagnosis Performed | COVID-19 Treatment |
Antifungals Used | Steroids? | Outcome after Treatment | References |
|---|---|---|---|---|---|---|---|---|
| Candidemia Candida duobushaemulonii Candida parapsilosis, Candida lusitaniae |
Elevated pro-inflammatory markers (d-dimer, ferritin, CRP, progressive thrombocytosis) and neutrophilia |
Acute pulmonary embolism with subarachnoid hemorrhage superimposed bacterial pneumonia |
CT scan, Culture, RT-PCR Blood, urine, and DTA |
Meropenem, Levofloxacin Trimethoprim/sulfamethoxazole, Amikacin, tigecycline, colistin |
Intravenous fluconazole |
NR | Dead | [31][32] |
| Candidemia (Candida glabrata) |
Leucocytes—normal, C-reactive protein and interleukin 6—altered |
Type-2 diabetes ischemic heart disease stadium IV, leg amputation highly suspected bacterial superinfection |
Chest X-ray and CT scan, RT-PCR, serology, MALDI-TOF |
Darunavir/ritonavir, HCQ, piperacillin/tazobactam, teicoplanin, ertapenem, colistin |
Caspofungin | NR | Dead | [32] |
| Co-Morbidity/ DiseaseModels |
Test/Diagnosis Performed | COVID-19 Treatment | Antifungals Used | Steroids? | Outcome after Treatment | References |
|---|
| Fungal Infection in COVID-19 Infection | Observed Immune Response | Co-morbidity/ Disease Models |
Test/Diagnosis Performed | COVID-19 Treatment | Antifungals Used | Steroids? | Outcome after Treatment | References |
|---|---|---|---|---|---|---|---|---|
| Obesity HT |
CT-scan, RT-PCR |
None mentioned | Linezolid, meropenem | NA | Died | [67] | ||
| Cryptococcus neoformans | High inflammatory response and immunosuppression | HAT, HBV | CT-scan, RT-PCR |
meropenem, vancomycin | Fluconazole | Yes (tacrolimus, prednisone) |
Death | [99][102] |
| Asthma HT DM |
CT-scan, RT-PCR | [ | 39 | ] | ||||
| Candidemia Candida auris (n = 10), Candida albicans (n = 3), Candida tropicalis (n = 1), Candida krusei (P. kudriavzevii) (n = 1) |
NA | Underlying chronic conditions (e.g., hypertension, n = 7; DM, n = 6; and chronic kidney and liver disease, n = 2) | MALDI-TOF and molecular identification—sequencing | NR | Micafungin | NR | Dead (n = 8) | [4] |
| Candidemia Candida auris (n = 3) |
NA | DM, hypertension, chronic renal failure, coronary artery disease, obesity |
Vitek 2 system, MALDI-TOF, sequencing, multiplex PCR |
NR | Anidulafungin | NR | Dead | [33][36] |
| Candidemia Candida auris (n = 12) |
NA | DM (n = 6), hypertension (n = 6), multiple myeloma (n = 1), stem cell transplantation (n = 1), dyslipidemia (n = 1), end stage renal disease (n = 1), bladder cancer (n = 1), obesity (n = 1), systematic lupus erythematosus (n = 1) |
PCR, MALDI-TOF, Vitek2, whole genome sequencing |
Remdesivir (n = 9), HCQ (n = 1), | Amphotericin B Micafungin, |
n = 10 | Dead (n = 6) Alive (n = 6) |
[34][40] |
2.2. Aspergillosis
| Fungal Infection in COVID-19 Infection | Observed Immune Response | Co-morbidity/ DiseaseModels |
Test/Diagnosis Performed | COVID-19 Treatment | Antifungals Used | Steroids? | Outcome after Treatment | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspergillosis Aspergillus spp., CAPA |
Highly permissive inflammatory response | DM, CVD | CT scan, Culture | HCQ | Azoles, liposomal amphotericin B | NR | Alive | [43] | ||||||||
| Immunocompromised | ARD, HT | Remdesivir | Amphotericin B | NA | Died | [68] | ||||||||||
| Acquired immunodeficiency and immunosuppression | HIV | CT scan, RT-PCR, Culture, ELISA | CT-scan, NR |
RT-PCR | Tenofovir-DF/Voriconazole | Emtricitabine-atazanavir/ritonavirYes (n = 7) |
Some alive and some dead | [44] | ||||||||
| Amphotericin B deoxycholate plus fluconazole | No | Death | [ | 100 | ] | [ | 103] | Aspergillosis Aspergillus fumigatus, CAPA |
Immunocompromised | DM Vascular diseaseDM, HT |
CT scan, Culture | NR | CT-scan, RT-PCR |
Tocilizumab, methylprednisolone, dexamethasone | Amphotericin B | Methylprednisolone, dexamethasone |
| High inflammatory response and immunosuppression | Stage IV prostate cancer HT, colon-sigma diverticulosis |
CT-scan | No | Fluconazole | Isavuconazole, voriconazole | No |
Died | Amphotericin B plus flucytosine[69Alive | ][42] | |||||||
| Dexamethasone | Death | [ | 101 | ] | [ | 104 | ] | HT, coronary heart disease, obesity | CT scan, RT-PCR, Culture, | HCQ, meropenem, azithromycin | Voriconazole | Yes | Dead | HT | CT-scan, RT-PCR[ |
Hydrocortisone26] |
| Amphotericin B | Hydrocortisone | |||||||||||||||
| High inflammatory response and immunosuppression | HT, DM | NA | Died | but COVID19 positive mentioned |
Tocilizumab and corticosteroids | Anidulafungin, Amphotericin,[70] |
||||||||||
| flucytosine | Methylprednisolone | Death | [ | 98 | ] | Low B-cell and T-cell response | Severe dyspnea, hypertension, DM | CT scan, RT-PCR, Serology | RD, multiple antibiotics | Multiple antifungals | No | Alive | [ | NA45] | ||
| CT-scan, | RT-PCR |
Remdesivir, tocilizumad, dexamethasone | Amphotericin B | Dexamethasone | Died | |||||||||||
| Coccidioidomycosis (Coccidioides immitis, C. posadasii) | Impaired cytokine signaling from CD4+ Th1 and cytotoxic CD8+ T-cells among patients | No associated respiratory symptoms & disease | [ | 71 | ] | |||||||||||
| CT scan, Culture, Serology | NR | Liposomal Amphotericin B | No | Alive | [ | 102 | ] | [ | Systemic pro-inflammatory cytokine responses | Asthma, DM, Myeloma | CT scan, RT-PCR, Culture, | NR | Voriconazole, isavuconazole, liposomal amphotericin B, caspofungin, anidulafungin | Yes | Some alive and some dead | [46] |
| High inflammatory response and immunosuppression | ALL, AML | RT-PCR, CT scan, Culture, Serology | NR | Caspofungin, fluconazole, liposomal amphotericin B, caspofungin, itraconazole | No | Some alive and some dead | [47] | |||||||||
| Aspergillosis Aspergillus spp., IA |
Acquired immunodeficiency and immunosuppression | ARD | Antigen, CT scan, Culture, Serology | NR | NR | Yes | Death (quick evolution) |
[48] | ||||||||
| Strong deregulation of core components of innate immune and inflammatory responses | RHAEM | NA | NA | NA | NA | NR | [49] |
2.3. Histoplasmosis
| Fungal Infection in COVID-19 Infection | Observed Immune Response | Co-morbidity/ Disease Models |
Test/Diagnosis Performed | COVID-19 Treatment | Antifungals Used | Steroids? | Outcome after Treatment | References |
|---|---|---|---|---|---|---|---|---|
| Histoplasmosis Histoplasma capsulatum |
Acquired immunodeficiency | HIV | CT-scan, RT-PCR |
Tenofovir/lamivudine and atazanavir/ritonavir ceftriaxone, azithromycin |
Itraconazole | Yes (dexamethasone) |
Alive | [27][52][27,52] |
| HIV | HIV | CT-scan, RT-PCR |
Atazanavir/ritonavir, tenofovir/emtricitabine | Itraconazole, amphotericin B deoxycholate |
No | Alive | [27] | |
| Inflammatory response | NA | CT-scan, RT-PCR |
Levofloxacin | Itraconazole | Yes (methylprednisolone) |
Alive | [53] | |
| NA | NA | CT scan, RT-PCR |
NA | Itraconazole | No | Alive | [54] | |
| Histoplasmosis Histoplasma capsulatum-like intracellular yeasts |
Acquired immunodeficiency | HIV | CT-scan, RT-PCR |
HCQ, lopinavir/ritonavir, tenofovir disoproxil fumarate/emtricitabine plus dolutegravir | Amphotericin B deoxycholate, itraconazole |
No | Lost to follow-up | [55] |
2.4. Mucormycosis
| 105 |
| ] | ||||||||||||||
| Asthma | ||||||||||||||
| HT | ||||||||||||||
DM |
CT-scan, RT-PCR |
Remdesivir, dexamethasone | Amphotericin B | Dexamethasone | Died | [72] | ||||||||
| HT | ||||||||||||||
| Coccidioidomycosis (Coccidioides immitis) | Depressed cellular immunity | Progressive respiratory symptoms, hypoxemia | CT scan, Culture, | Remdesivir | Fluconazole | No | Alive | [103][106] | CT-scan, RT-PCR |
HCQ, lopinavir–ritonavir | Amphotericin B | NA | Died | [73] |
| Pneumocystis jirovecii | Cytokine release storm | RA | CT scan, Culture, Serology | HCQ, Tocilizumab | Caspofungin, ganciclovir, cefoperazone | Glucocorticoids | NR | [104][107] | DM ICM RD |
CT-scan, RT-PCR |
Meropenem | |||
| Functional immune suppression related to CD4+ lymphocytopenia | HIV, progressive hypoxemia | RT-PCR, Culture, Serology, CT | Amphotericin B | Dexamethasone | Alive | NR | Trimethoprim- sulfamethoxazole[74] | |||||||
| NR | NR | [ | 105 | ] | [ | 108 | ] | DM | CT-scan, RT-PCR |
NA | Amphotericin B | NA | Alive | |
| Immunocompromised | ARD, DM, HT | [ | RT-PCR, Culture, Serology, | 75 | ] | |||||||||
| HCQ, Lopinavir-ritonavir | Antifungals and antibacterials | Yes | Some alive and some dead | [ | 106 | ] | [109] | HT, DM |
CT-scan, RT-PCR |
NA | Liposomal amphotericin B, itraconazole | NA | Alive | [76] |
| Low CD4 count (35.6%) | HIV | CT, RT-PCR, Multiplex PCR | NR | Co-trimoxazole and oral prednisolone | No | Alive | [107][110] | NA | RT-PCR CT-scan |
Remdesivir, dexamethasone, metformin, glipizide | Amphotericin B, ceftriaxone | Dexamethasone | Live | [77 |
| Anemia, lymphopenia, raised C-reactive protein, immunosuppression | ] | |||||||||||||
| HIV | CT, RT-PCR | NR | Co-trimoxazole, IV pentamidine | No | Death | [ | 108][111] | DM | CT-scan, RT-PCR |
|||||
| Severe depletion of CD4+ cells | Meropenem, oseltamivir | tocilizumab, sitagliptin/metformin |
Amphotericin B | Methylprednisolone, | HIV dexamethasone |
Died | [69] | |||||||
| RT-PCR, Culture, CT | Emtricitabine, Ritonavir | Trimethoprim-sulfamethoxazole | No | NR | [ | 109 | ][99] | DM | CT-scan, RT-PCR |
Remdesivir, ceftriaxone, azithromycin, dexamethasone | Voriconazole, liposomal amphotericin B | Dexamethasone | Live | [78] |
| Immunocompetent patient | Recovered from COVID-19 | RT-PCR, Culture, CT | Enoxaparin, ceftaroline | Trimethoprim-sulfamethoxazole, methylprednisolone | Yes | Alive | [110][100] | DM (1 patient) No co-morbidity (1 patient) |
CT-scan | |||||
| Immunocompromised patients | Remdesivir, convalescent plasma, | vancomycin, piperacillin-tazobactam |
HT, hepatic steatosis, massive lung thrombosesAmphotericin B | NA | Live (n = 1) Died n = (1) |
RT-PCR, Culture, CT, Histopathology[68] | ||||||||
| Remdesivir | Trimethoprim-sulfamethoxazole, prednisone | Yes | Some alive and some dead | [ | 111 | ] | [101] | Obesity DM |
CT-scan, | |||||
| Saccharomyces cerevisiae (boulardii) (n = 2) | RT-PCR |
Amoxicillin-clavulanate, imipenem/linezolid |
Amphotericin B | ImmunosuppressionNA | Died | [79] | ||||||||
| HT (first) | Diabetes (Second) | RT-PCR | Oseltamivir HCQ |
Anidulafungin, fluconazole |
No treated with Ultra-Levure [preparation of Saccharomyces cerevisiae (boulardii)] |
Both live | [112] | DM (n = 8) CRF (n = 3) |
CT-scan | |||||
| Fusarium proliferatum | Broad-spectrum antibiotics | immunocompetent diabetic patient | HATLiposomal amphotericin B | Dexamethasone |
substituted hypothyroidism | RT-PCR | NoDied (n = 7) Alive (n = 4) |
[80] | ||||||
| Amphotericin B | caspofungin | No | Live | [ | 113 | DM HT (all patients) |
RT-PCR | HCQ, glucocorticoids |
Systemic antifungals | Glucocorticoids | Died (n = 7) Live (n = 8) |
[81] | ||
| T2DM (4) T2DM with HT (1) HT (1) Kidney Disease (1) |
CT-scan, RT-PCR |
Tocilizumab, prednisolone, piperacillin/tazobac, linezolid |
Voriconazole | Prednisolone | Died (n = 3) Alive (n = 4) |
[82] | ||||||||
| ] | DM (21-cases) HT (14-cases) Renal failure (1-case) |
CT-scan, RT-PCR |
HCQ, azithromycin | Caspofungin | Combination of steroids | All Live | [76] | |||||||
| DM (16) | RT-PCR | Corticosteroids | Liposomal amphotericin B, voriconazole, posaconazole |
On Steroid | Alive (n = 10) Died n = (6) |
[83] | ||||||||
| HT, UTI |
CT-scan, RT-PCR |
Either dexamethasone or methylprednisolone (7 patients); interferon (2 patient); remdesivir (1 patient); flavipiravir and HCQ (1 patient) |
Amphotericin B, posaconazole | Dexamethasone or Methylprednisolone (n = 7) |
Live | [84] | ||||||||
| DM | RT-PCR CT-scan |
Remdesivir, levofloxacin, dexamethasone, meropenem, vancomycin, piperacillin/tazobactam | Amphotericin B, posaconazole | Dexamethasone | Live | [85] | ||||||||
| No co-morbidity | CT-scan, RT-PCR |
HCQ | Amphotericin B | NA | Died | [86] | ||||||||
| chronic lymphocytic leukemia DM |
RT-PCR | NA | Amphotericin B | NA | Died | [87] | ||||||||
| DM HT asthma |
RT-PCR | NA | Amphotericin B | No | Died | [88] | ||||||||
| AML | CT-scan, RT-PCR |
HCQ lopinavir-ritonavir |
Amphotericin B | NA | Died | [73] | ||||||||
| renal disease | CT-scan, RT-PCR |
Remdesivir, vancomycin, cefepime | Liposomal amphotericin B, posaconazole | Dexamethasone | Died | [72] | ||||||||
| ICM HF s/p OHT DM HT CKD |
RT-PCR | Remdesivir methylprednisolone |
Fluconazole | Methylprednisolone, dexamethasone |
Died | [89] | ||||||||
| No history of any co-morbidity | CT-scan, RT-PCR |
Tocilizumab | Liposomal amphotericin B, posaconazole, isavuconazole | Dexamethasone | Live | [90] | ||||||||
| DM HT |
Piperacillin/tazobactam, HCQ, azithromycinlopin, vir/ritonavir, prednisone Dexamethasone |
Liposomal amphotericin B, isavuconazole, posaconazole | Prednisone, Dexamethasone | Live | [91] | |||||||||
| HT | RT-PCR | Remdesivir, dexamethasone | Amphotericin B | Dexamethasone | Died | [92] | ||||||||
| T2DM (all 6 patients) |
CT-scan, RT-PCR |
Prednisolone, dexamethasone, methylprednisolone | Amphotericin B, posaconazole | Prednisolone, Dexamethasone, methylprednisolone |
All Live |
[93] | ||||||||
| DM HT |
CT-scan, RT-PCR |
Remdesivir, interferon-alpha | Systemic antifungals | Systemic corticosteroid | Died | [94] | ||||||||
| T2DM, HT (2) T2DM (3) |
CT-scan, RT-PCR |
Tocilizumab, convalescent plasma, methylprednisolone |
Liposomal amphotericin B, posaconazole |
Methylprednisolone | Died (n = 2) Alive (n = 3) |
[95] | ||||||||
| T1DM | CT-scan, RT-PCR |
Ceftriaxone, azithromycin, dexamethasone, remdesivir, tocilizumab |
Amphotericin B | Dexamethasone | Live | [71] | ||||||||
| Obesity hypothyroidism |
CT-scan, RT-PCR |
HCQ, remdesivir, vancomycin, meropenem | Liposomal amphotericin B, posaconazole |
Prednisone | Died | [96] | ||||||||
| HT Asthma |
RT-PCR | Meropenem, remdesivir, dexamethasone | Liposomal amphotericin B | Dexamethasone, prednisolone | Died | [97] |
