Black phosphorus (BP) is one of the emerging versatile nanomaterials with outstanding biocompatibility and biodegradability, exhibiting great potential as a promising inorganic nanomaterial in the biomedical field. BP nanomaterials possess excellent ability for valid bio-conjugation and molecular loading in anticancer therapy. Generally, BP nanomaterials can be classified into BP nanosheets (BPNSs) and BP quantum dots (BPQDs), and both of them can be oxidized and degraded to nontoxic phosphonates and phosphate under physiological conditions, improving their safety as a nano drug carrier in cancer therapy. In addition, BP nanomaterials can be applied as photothermal agents (PTA) for photothermal therapy (PTT) and photodynamic therapy (PDT) due to their high photothermal conversion efficiency, large extinction coefficients, and capability of producing singlet oxygen. Recently, it has been reported that BP-based phototherapy is capable of activating immune responses and alleviating the immunosuppressive tumor microenvironment by detection of T lymphocytes and various immunocytokines, indicating that BP-based nanocomposites not only serve as effective PTAs to ablate large solid tumors but also function as an immunomodulation agent to eliminate discrete tumorlets.
- black phosphorus nanomaterial
- synergistic therapeutic modality
- cancer immunotherapy
- phototherapy
- immune stimulation
1. Introduction
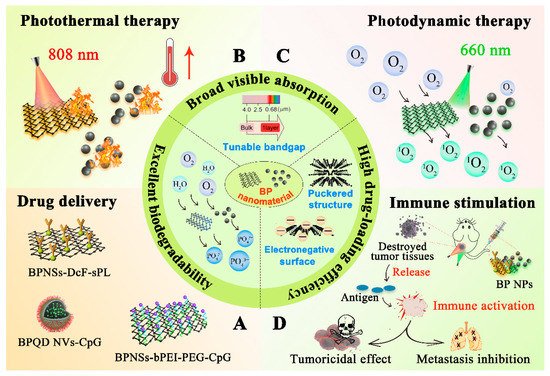
2. Properties of Black Phosphorus Nanomaterials
2.1. Optical Properties
2.2. Biocompatibility and Biodegradability
2.3. High Loading Efficiency
References
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019, 54, 407–419.
- Jain, R.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664.
- Mei, Y.; Tang, L.; Xiao, Q.; Zhang, Z.; Zhang, Z.; Zang, J.; Zhou, J.; Wang, Y.; Wang, W.; Ren, M. Reconstituted high density lipoprotein (rHDL), a versatile drug delivery nanoplatform for tumor targeted therapy. J. Mater. Chem. B 2021, 9, 612–633.
- Yang, Z.; Ma, Y.; Zhao, H.; Yuan, Y.; Kim, B. Nanotechnology platforms for cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1590.
- Li, J.; Han, M.; Li, J.; Ge, Z.; Wang, Q.; Zhou, K.; Yin, X. Sterically stabilized recombined HDL composed of modified apolipoprotein A-I for efficient targeting toward glioma cells. Drug Deliv. 2020, 27, 530–541.
- Kummerer, K.; Menz, J.; Schubert, T.; Thielemans, W. Biodegradability of organic nanoparticles in the aqueous environment. Chemosphere 2011, 82, 1387–1392.
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016, 308, 328–334.
- Sun, L.; Li, M.; Sun, K.; Yu, S.; Wang, R.; Xie, H. Electrochemical Activity of Black Phosphorus as an Anode Material for Lithium-Ion Batteries. J. Phys. Chem. C 2012, 116, 14772–14779.
- Li, L.; Yu, Y.; Ye, G.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.; Zhang, Y. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377.
- Chen, X.; Ponraj, J.; Fan, D.; Zhang, H. An overview of the optical properties and applications of black phosphorus. Nanoscale 2020, 12, 3513–3534.
- Fang, Y.; Ge, Y.; Wang, C.; Zhang, H. Mid-Infrared Photonics Using 2D Materials: Status and Challenges. Laser Photonics Rev. 2020, 14.
- Qiu, M.; Ren, W.; Jeong, T.; Won, M.; Park, G.; Sang, D.; Liu, L.; Zhang, H.; Kim, J. Omnipotent phosphorene: A next-generation, two-dimensional nanoplatform for multidisciplinary biomedical applications. Chem. Soc. Rev. 2018, 47, 5588–5601.
- Li, Z.; Zhang, X.; Ouyang, J.; Chu, D.; Han, F.; Shi, L.; Liu, R.; Guo, Z.; Gu, G.; Tao, W.; et al. Ca2+-supplying black phosphorus-based scaffolds fabricated with microfluidic technology for osteogenesis. Bioact. Mater. 2021, 6, 4053–4064.
- Lu, F.; Li, Z.; Kang, Y.; Su, Z.; Yu, R.; Zhang, S. Black phosphorus quantum dots encapsulated in anionic waterborne polyurethane nanoparticles for enhancing stability and reactive oxygen species generation for cancer PDT/PTT therapy. J. Mater. Chem. B 2020, 8, 10650–10661.
- Li, Q.; Wu, J.; Liu, Y.; Qi, X.; Jin, H.; Yang, C.; Liu, J.; Li, G.; He, Q. Recent advances in black phosphorus-based electrochemical sensors: A review. Anal. Chim. Acta 2021, 1170.
- Zhang, D.; Lin, Z.; Lan, S.; Sun, H.; Zeng, Y.; Liu, X. The design of Janus black phosphorus quantum [email protected] nanoparticles for simultaneously enhancing environmental stability and photodynamic therapy efficiency. Mater. Chem. Front. 2019, 3, 656–663.
- Zeng, G.; Chen, Y. Surface modification of black phosphorus-based nanomaterials in biomedical applications: Strategies and recent advances. Acta Biomater. 2020, 118, 1–17.
- Liu, W.; Tao, Z.; Wang, D.; Liu, Q.; Wu, H.; Lan, S.; Dong, A. Engineering a black phosphorus-based magnetic nanosystem armed with antibacterial N-halamine polymer for recyclable blood disinfection. Chem. Eng. J. 2021, 415.
- Li, Y.; Li, X.; Zhou, F.; Doughty, A.; Hoover, A.; Nordquist, R.; Chen, W. Nanotechnology-based photoimmunological therapies for cancer. Cancer Lett. 2019, 442, 429–438.
- Sanmamed, M.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326.
- Beatty, G.; Gladney, W. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 2015, 21, 687–692.
- Chen, W.; Singhal, A.; Liu, H.; Nordquist, R. Antitumor immunity induced by laser immunotherapy and its adoptive transfer. Cancer Res. 2001, 61, 459–461.
- Chen, W.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.; Liu, Z.; Han, Y.; Wang, L.; Li, J.; et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv. Mater. 2017, 29.
- Yang, X.; Wang, D.; Shi, Y.; Zou, J.; Zhao, Q.; Zhang, Q.; Huang, W.; Shao, J.; Xie, X.; Dong, X. Black Phosphorus Nanosheets Immobilizing Ce6 for Imaging-Guided Photothermal/Photodynamic Cancer Therapy (vol 10, pg 12431, 2018). ACS Appl. Mater. Interfaces 2019, 11, 43797.
- Xu, X.; Lu, H.; Lee, R. Near Infrared Light Triggered Photo/Immuno-Therapy Toward Cancers. Front. Bioeng. Biotechnol. 2020, 8, 488.
- Li, X.; Lovell, J.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674.
- Kobayashi, H.; Furusawa, A.; Rosenberg, A.; Choyke, P. Near-infrared photoimmunotherapy of cancer: A new approach that kills cancer cells and enhances anti-cancer host immunity. Int. Immunol. 2021, 33, 7–15.
- Li, Y.; Liu, Z.; Hou, Y.; Yang, G.; Fei, X.; Zhao, H.; Guo, Y.; Su, C.; Wang, Z.; Zhong, H.; et al. Multifunctional Nanoplatform Based on Black Phosphorus Quantum Dots for Bioimaging and Photodynamic/Photothermal Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 25098–25106.
- Liu, W.; Dong, A.; Wang, B.; Zhang, H. Current Advances in Black Phosphorus-Based Drug Delivery Systems for Cancer Therapy. Adv. Sci. 2021, 8, 2003033.
- Hanlon, D.; Backes, C.; Doherty, E.; Cucinotta, C.; Berner, N.; Boland, C.; Lee, K.; Harvey, A.; Lynch, P.; Gholamvand, Z.; et al. Liquid exfoliation of solvent-stabilized few-layer black phosphorus for applications beyond electronics. Nat. Commun. 2015, 6, 8563.
- Gao, N.; Xing, C.; Wang, H.; Feng, L.; Zeng, X.; Mei, L.; Peng, Z. pH-Responsive Dual Drug-Loaded Nanocarriers Based on Poly (2-Ethyl-2-Oxazoline) Modified Black Phosphorus Nanosheets for Cancer Chemo/Photothermal Therapy. Front. Pharmacol. 2019, 10, 270.
- Zhang, X.; Tang, J.; Li, C.; Lu, Y.; Cheng, L.; Liu, J. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact. Mater. 2020, 6, 472–489.
- Wang, H.; Yang, X.; Shao, W.; Chen, S.; Xie, J.; Zhang, X.; Wang, J.; Xie, Y. Ultrathin Black Phosphorus Nanosheets for Efficient Singlet Oxygen Generation. J. Am. Chem. Soc. 2015, 137, 11376–11382.
- Zhang, W.; Yu, L.; Jiang, Y.; Guo, C. Phycocyanin-functionalized black phosphorus quantum dots enhance PDT/PTT therapy by inducing ROS and irreparable DNA damage. Biomater. Sci. 2021, 9, 5302–5318.
- Shao, J.; Xie, H.; Huang, H.; Li, Z.; Sun, Z.; Xu, Y.; Xiao, Q.; Yu, X.; Zhao, Y.; Zhang, H.; et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 2016, 7, 12967.
- Liu, X.; Chen, K.; Li, X.; Xu, Q.; Weng, J.; Xu, J. Electron Matters: Recent Advances in Passivation and Applications of Black Phosphorus. Adv. Mater. 2021, 2005924.
- Wang, Z.; Liu, Z.; Su, C.; Yang, B.; Fei, X.; Li, Y.; Hou, Y.; Zhao, H.; Guo, Y.; Zhuang, Z.; et al. Biodegradable Black Phosphorus-based Nanomaterials in Biomedicine: Theranostic Applications. Curr. Med. Chem. 2019, 26, 1788–1805.
- Cai, Y.; Tang, R. Calcium phosphate nanoparticles in biomineralization and biomaterials. J. Mater. Chem. 2008, 18, 3775–3787.
- Zhou, W.; Pan, T.; Cui, H.; Zhao, Z.; Chu, P.; Yu, X. Black Phosphorus: Bioactive Nanomaterials with Inherent and Selective Chemotherapeutic Effects. Angew. Chem. Int. Ed. 2019, 58, 769–774.
- Abbaraju, P.; Meka, A.; Song, H.; Yang, Y.; Jambhrunkar, M.; Zhang, J.; Xu, C.; Yu, M.; Yu, C. Asymmetric Silica Nanoparticles with Tunable Head–Tail Structures Enhance Hemocompatibility and Maturation of Immune Cells. J. Am. Chem. Soc. 2017, 139, 6321–6328.
- Xie, Z.; Wang, D.; Fan, T.; Xing, C.; Li, Z.; Tao, W.; Liu, L.; Bao, S.; Fan, D.; Zhang, H. Black phosphorus analogue tin sulfide nanosheets: Synthesis and application as near-infrared photothermal agents and drug delivery platforms for cancer therapy. J. Mater. Chem. B 2018, 6, 4747–4755.
- Zhou, W.; Cui, H.; Ying, L.; Yu, X. Enhanced Cytosolic Delivery and Release of CRISPR/Cas9 by Black Phosphorus Nanosheets for Genome Editing. Angew. Chem. Int. Ed. 2018, 57, 10268–10272.
