1. Introduction
Pregnancy causes significant unique changes in maternal immune responses and metabolism, yet it is unclear whether/how these alterations may be connected to infections [1]. There is however, strong and solid scientific evidence that pregnancy can induce bacterial dysbiosis, especially in the vaginal and gut microbiome, leading to metabolic alterations and complications in the mother and the newborn [1][2][3][1,2,3]. Hypertension is the most frequent health complication in pregnancy, affecting 10% of pregnancies worldwide [4]. Classification of hypertensive disorders during pregnancy are classified into 4 categories including pre-eclampsia superimposed on chronic hypertension and pre-eclampsia-eclampsia [5].
Pre-eclampsia is the second most frequent direct source of maternal mortality, causing an average of 500,000 fetal and neonatal deaths and an estimated of 76,000 direct maternal deaths and each year [6]. Although the causes of pre-eclampsia are still discussed, research has suggested that the placenta has a central place in the pathogenesis of this disease. Evidence supports that fetuses and neonates of preeclamptic women are especially impacted by the maternal condition, independently of uteroplacental restriction of flow [7][8]. Pre-eclampsia is considered to be an endothelial disturbance where the oxidative pathophysiological stress and disturbance of lipid status have potential implications in the development of pre-eclampsia among high-risk pregnancies [8][9].
2. Hormonal Oral, Immunological and Oral Microbiota Changes and Their Impact on Periodontal Disease during the Pregnancy
During gestation, the female body undergoes a series of hormonal, metabolic, and immunological changes
[9][10][38,39], which may have a significant effect on the composition of the oral microbiome.
During pregnancy, the level of hormones changes
[11][40]. Particularly, the increase of estrogen and progesterone can increase her susceptibility to bacterial plaque provoking the apparition gingivitis that is most frequent during the second to the third trimester of pregnancy
[12][41]. The analysis of salivary estrogen levels showed that the estradiol level was ten times higher in pregnant women in the first trimester than non-pregnant women
[13][42]. Salivary estrogen levels increased significantly during the second and third trimesters. In both participant groups, the bleeding on probing was correlated significantly with plaque index, but not with estrogen levels. In all trimesters and postpartum, subjects with high estrogen and PI levels had a higher frequency of gingivitis in pregnancy. During the second and third trimesters, the simultaneous increase in estrogen and PI levels increase the risk of developing gingivitis compared to PI alone. Therefore, during pregnancy, estrogen level determines the magnitude of gingival inflammation developed against microbial plaque at the gingival margin
[13][42].
Progesterone, as its name suggests, is a pregnancy-promoting hormone. Progesterone levels throughout pregnancy increase progressively, reaching concentrations that are ten times higher than those found during the luteal phase of the genital cycle
[14][43]. Gürsoy et al. demonstrated that salivary progesterone concentration increases significantly throughout pregnancy and decreases postpartum. Pregnant women have approximately 18 times higher progesterone level than non-pregnant women
[13][42].
Estrogens and progesterone perform their functions by binding to specific intracellular receptors involved in the regulation of cell growth, differentiation and development
[15][16][44,45]. Because estrogen receptor and progesterone receptor localization has been reported in the human periodontium, the increase in circulating levels of estrogen and progesterone should have a dramatic effect on the periodontium throughout pregnancy and correlates with the clinical phenomenon
[14][43].
The serum estradiol and progesterone levels increased greatly during pregnancy and were much higher in the pregnant women than in the nonpregnant group
[17][46]. A positive association was found between increased gingival inflammation and increased serum estradiol and progesterone levels during pregnancy
[17][46]. A positive association was also observed between the presence of periodontopathogen such as P. gingivalis and the progesterone levels in the first trimester
[12][41]. The hormonal modifications promote the growth of several Gram-negative anaerobic bacteria in the oral cavity such as Prevotella intermedia (P. intermedia), Prevotella nigrescens and Campylobacter rectus (C. rectus)
[18][10].
The periodontium is a target tissue for estrogens and progesterone that cause vascular, cellular and microbiological changes
[19][20][47,48]. The elevated levels of estrogens and progesterone act on the gingival vasculature and could cause an increase of erythema, edema, crevicular fluid, and bleeding
[12][41]. Moreover, progesterone increases the synthesis of prostaglandins, particularly prostaglandin E2. Prostaglandins increase vascular capillarity and permeability, thus amplifying the clinical manifestations of gingival inflammation, the gingiva exudates, and this exudate allows bacteria to multiply
[20][48]. Progesterone also retards the synthesis of glycosaminoglycans by gingival fibroblasts and thus acts on the inflammatory reaction
[21][49]. On the other hand, estrogens reduce the keratinization of the gingival epithelium and alter the fundamental substance of the connective tissue
[22][50]. The decrease in epithelial keratinization associated with the increase in epithelial glycogen leads to a decrease in the effectiveness of the epithelial barrier
[23][51].
During pregnancy, the immune system is modified to be able to tolerate the fetus. This modification affects the defensive system of periodontal tissues
[19][47]. The increase of sex hormones acts on the function and activity of polymorphonuclear. Impaired neutrophil functions are associated to an increased susceptibility to inflammation
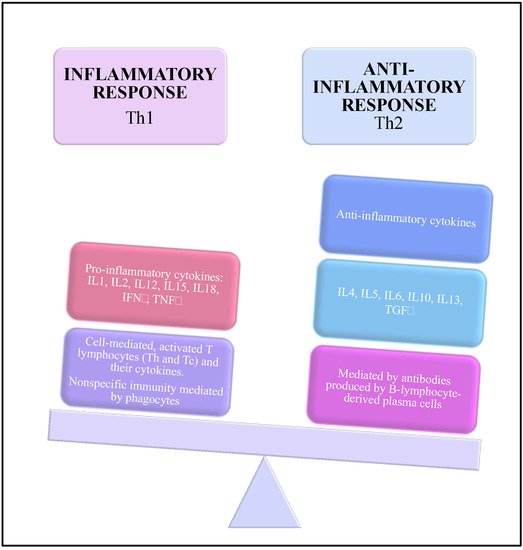
[24][28]. The environment is in an anti-inflammatory state controlled by anti-inflammatory cytokines such as interleukin- (IL) 4, IL-5, IL-10, IL-13, and granulocyte-macrophage stimulating factor
[25][52]. This anti-inflammatory state can be dysregulated and associated to a shift toward the pro-inflammatory cytokines represented by IL-1, IL-2, IL-6, IL-12, IL-15, IL-18, interferon-γ, and tumor-necrosis factor-α
[26][27][53,54].
At the systemic level, the immune response of the pregnant woman is associated with an alteration of the balance between cellular immunity (Th1 cytokines) and humoral immunity (Th2 cytokines). Humoral immunity increases while cellular immunity decreases
[19][47] (
Figure 1).
Figure 1. Immunological changes during the pregnancy.
Receptors for estrogen have been found in thymocytes and thymic epithelial cells. Estrogen injection is followed by atrophy of the thymus and, therefore, the number of TCD4 and TCD8 lymphocytes is reduced
[28][55]. Also, intracellular and membrane estrogen receptors have been reported in these lymphocytes, so that estrogens reduce the number of TCD4+ and TCD8+ lymphocytes and increase the activity of B lymphocytes, as well as the production of immunoglobulin M and G
[29][56].
Several studies have analyzed the association between the quantity of bacteria (PI) and the gingivitis. In a meta-analysis, Figuero et al. selected 7 studies for analysis of PI
[30][24]. In cohort studies, PI was not significantly modified during pregnancy, but in cross-sectional studies, PI was significantly slightly higher in pregnant women. No differences were found when comparing pregnant and postpartum women. These results are confirmed by the study of Wu et al.
[17][46]. They used a methodology very similar to that of Gürsoy et al.
[31][57], except that participants had excellent plaque control, obtained through oral hygiene instructions throughout the duration of the study. They reported that PI was not significantly modified, as gingival index and bleeding increased significantly in the second and third trimester of pregnancy.
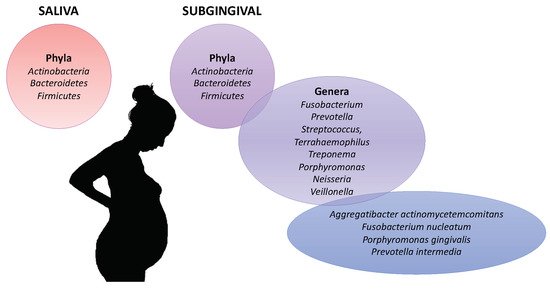
The oral microbiota was compared between pregnant and non-pregnant women (
Figure 2)
[32][33][34][58,59,60]. Fujiwara et al. analyzed by polymerase chain reaction (PCR) the subgingival microbiota of 132 Japanese pregnant women and 51 Japanese nonpregnant women
[34][60]. The total number of microorganisms was significantly higher in the saliva of pregnant women compared to Japanese non-pregnant
[34][60]. Their microbiota contained higher amounts of P. gingivalis, Aa in early and mid-pregnancy. P. intermedia and F. nucleatum did not change depending on whether women were pregnant or not. Candida species were more prevalent in mid and late gestation
[34][60]. Borgo et al.
[32][58] analyzed by quantitative PCR the presence of A. actinomycetemcomitans, P. intermedia, P. gingivalis and F. nucleatum in 23 pregnant or 9 non-pregnant women. They showed that Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) was detected in significant higher amounts in the second trimester and in the third trimester of gestation
[32][58]. F. nucleatum and P. intermedia was detected in high level in pregnant women whereas P. gingivalis was detected in both pregnant and non-pregnant
[32][58]. Lin et al. observed in pregnant women, a higher abundance of Treponema, Porphyromonas and Neisseria, while in the non-pregnant women, Streptococcus and Veillonella were more abundant
[35][61]. Balan et al. also observed that the oral microbiota was composed of a higher abundance of pathogenic species (Prevotella, P. gingivalis and F. nucleatum) in healthy pregnant as compared with nonpregnant one whereas they had similar gingival and plaque index scores
[36][62].
Figure 2. Oral microbiota associated with the pregnancy.
The evolution of the oral microbiota during the gestation was studied. In pregnant women, DiGiulio et al. observed that the average taxonomic composition of the saliva remained constant over gestational time
[37][63]. Balan et al. also observed that subgingival and saliva microbiota were relatively stable in terms of species richness and diversity during the course of pregnancy
[36][62]. However, they observed the increasing of pathogenic bacterial during pregnancy. In pregnant women, members of phyla Actinobacteria, Bacteroidetes and Firmicutes dominated in both saliva and subgingival samples. The genera Fusobacterium, Prevotella, Streptococcus, Terrahaemophilus and Veillonella were most abundant in subgingival microbiota. Concordant results were observed in pregnant Chinese women
[35][61]. During the pregnancy, a higher abundance of Prevotella species, P. gingivalis, and F. nucleatum was observed
[36][62].
The composition of the oral microbiota was also compared between pregnant women suffering or not of gingivitis. Yang et al., in a pilot study, concluded that the gingivitis in pregnant women was not correlated with a shift in the overall composition or diversity of the subgingival microbiota
[27][54]. The main phyla observed in the subgingival microbiota of both groups were Actinobacteria, Bacteroidetes, Firmicutes, Fusobacteria, Proteobacteria, and Spirochaetes. However, they observed modification of several bacteria taxa in pregnant women suffering o gingivitis compared to healthy pregnant women. Particularly, 5 operational taxonomic units contained species known as periodontal or opportunistic pathogens. These unbalance between commensal bacteria and pathogenic bacteria is associated, the dysbiosis of the subgingival microbiota and with the apparition of gingivitis
[38][64]. In 2014, Tellapragada et al. analyzed by PCR the subgingival plaque of pregnant women and demonstrated an association between gingivitis and the presence of C. rectus, P. gingivalis, P. intermedia, Parvimona nigrescens, and Treponema denticola (T. denticola)
[39][65].
After the delivery, the abundance of pathogenic species decreased was accompanied by simultaneous repopulation of healthy microbiome such as Lautropis mirabilis sp., Rothia aeria, Granulicatella adiacens, and SR1 sp
[36][62]. These species have been reported to be dominating in the health-associated microbial communities in healthy subjects in previous studies
[40][66]. Pregnancy-related transition to the pathogenic microbiome and its restoration to health during the postpartum period could be a result of complex host-microbial interactions that may be taking place under the influence of hormonal and immunological factors
[24][28]. In addition, pregnancy-induced perturbations in the oral cavity may disrupt the ecological balance maintained by interspecies interactions
[18][10]. These may trigger the overgrowth of species with pathogenic potential and suppress the healthy microbiome
[18][10].
3. Periodontal Pathogens and Pre-Eclampsia
In pregnant women, the PD and more particularly, the presence of periodontal pathogens has been associated to adverse pregnancy outcomes such as pre-eclampsia
[18][38][41][42][10,64,67,68]. Several meta-analyses concluded that maternal PD was an independent predictor of pre-eclampsia
[43][44][45][46][47][48][69,70,71,72,73,74]. Three pathways could explain this association
[49][38][12,64]. Firstly, periodontopathogen that are mobile bacteria could migrate, invade the epithelium, the connective tissue, reach the bloodstream and diffuse into the body. Thus, adhesion proteins expressed on the surface of the bacteria can bind to the placental cell receptors and trigger a downstream inflammatory response
[50][75]. Secondly, inflammatory molecules produced in response to periodontopathogens could migrate through the blood stream. Finally, periodontopathogens can reach and colonize the vaginal microbiota through the gastrointestinal tract or during oro-genital contacts
[18][38][10,64].
Pre-eclampsia is a placental dysfunction due to early angiogenic and inflammatory dysregulation. Clinically, it is characterized by the new appearance of hypertension, end organ dysfunction and potential proteinuria after 20 weeks of gestation. The consequences can be the maternal morbidity or adverse fetal outcomes such as intra-uterine growth restriction, preterm birth, placental abruption, fetal distress, and fetal death in utero
[51][76]. Even if the real cause of pre-eclampsia is not elucidated, it appears that dysbiosis of the placental microbiota could be a key risk factor
[52][53][77,78].
While in normotensive women, no bacteria could be detected by PCR in placental samples, 12.7% of samples from women with pre-eclampsia had bacteria. The bacteria identified included Bacillus cereus, Escherichia, Listeria, Salmonella (usually associated with gastrointestinal infection); Anoxy bacillus and Klebsiella pneumonia (usually associated with respiratory tract infections); and Dialister, Porphyromonas, Prevotella, and Variovorax (usually associated with periodontitis)
[54][79]. The comparative metagenomic analysis on 320 placental specimens revealed that the placental microbiome was closely related to the supragingival plaque
[53][78]. Concordant results were obtained by Barak et al.
[55][80]. 50% of placenta samples from pregnant women suffering of pre-eclampsia had periodontopathogens such as Actinobacillus actinomycestemcomitans, F. nucleatum, P. gingivalis, P. intermedia, Tannerella forsynthia, and T. denticola (Spirochaetes) in the placentas of women with pre-eclampsia.
The presence of periodonthopathogens that induce PD conduce to the increase of pro-inflammatory cytokines and thus the dysregulation of the normal immunological state during pregnancy
[56][81]. Indeed, pregnant women suffering from PD presented a high level of plasma C-reactive protein that is the witness of systemic inflammation
[27][54]. An increase of the level of pro-inflammatory cytokines in the peripheral circulation was also observed in case of PD
[57][82]. In pre-eclamptic women with periodontitis, the presence of P. gingivalis and P. intermedia was associated with the increase of TLR-4 and NF-κB expression in the placenta
[58][83].
In addition, during placental development, to ensure androgen signaling and epigenetic regulation of gene expression, the placenta utilizes histone lysine demethylases and androgen receptors
[59][84]. Thus, disruption of androgens could be the cause of abnormal placental development. And since a study showed that P. intermedia and P. gingivalis can reduce testosterone to 5-alpha dihydrotestosterone (DHT) and induce DHT synthesis by fibroblasts
[60][85] then these bacteria could interfere with androgen signaling and deregulate placental development.
Periodontopathogen could also be pathogens of the placenta bed. The favorable outcome of pregnancy is associated with uterine vascular changes with, in particular, the adequate remodeling of the uterine spiral arteries. Inappropriate remodeling of the myometrial segments of the uterine spiral arteries is termed defective deep placentation
[61][62][86,87]. The defective deep placentation has been demonstrated in case of pre-eclampsia
[62][87]. During the first trimester of pregnancy, extravillous trophoblasts form anchoring villi in order to attach the placenta to the decidua and the uterine wall which will allow the transmission of pathogens from mother to fetus
[63][88]. The presence of P. gingivalis was detected in 70–92% of samples from the inner third of the placental bed - known as the decidua - of women with pre-eclampsia
[64][65][89,90]. P. gingivalis was detected within the villous stroma or umbilical cord. Its presence in the umbilical cord was significantly associated with pre-eclampsia
[66][91]. Several in vitro and in vivo studies realized in rat or primate’ models indicate that P. gingivalis can impact the remodeling of the uterine spiral arteries
[67][68][69][92,93,94].
4. Conclusions
We conclude that fluctuating progesterone and estrogen levels and the modification of the immune response during pregnancy impact the subgingival microbiota and contribute to increase the risk of apparition of PD. Moreover, the PD and more particularly, the periodontal pathogens increase the risk of pre-eclampsia.