The human body is highly complex and comprises a variety of living cells and extracellular material, which forms tissues, organs, and organ systems. Human cells tend to turn over readily to maintain homeostasis in tissues. However, postmitotic nerve cells exceptionally have an ability to regenerate and be sustained for the entire life of an individual, to safeguard the physiological functioning of the central nervous system. For efficient functioning of the CNS, neuronal death is essential, but extreme loss of neurons diminishes the functioning of the nervous system and leads to the onset of neurodegenerative diseases. Neurodegenerative diseases range from acute to chronic severe life-altering conditions like Parkinson’s disease and Alzheimer’s disease. Millions of individuals worldwide are suffering from neurodegenerative disorders with little or negligible treatment available, thereby leading to a decline in their quality of life. Neuropathological studies have identified a series of factors that explain the etiology of neuronal degradation and its progression in neurodegenerative disease. The onset of neurological diseases depends on a combination of factors that causes a disruption of neurons, such as environmental, biological, physiological, and genetic factors.
Neurodegeneration is the progressive loss of structure or function of neurons, which may ultimately involve cell death. Many neurodegenerative diseases—such as amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, Alzheimer's disease, Huntington's disease, and prion diseases—occur as a result of neurodegenerative processes. Neurodegeneration can be found in the brain at many different levels of neuronal circuitry, ranging from molecular to systemic. Because there is no known way to reverse the progressive degeneration of neurons, these diseases are considered to be incurable. Biomedical research has revealed many similarities between these diseases at the sub-cellular level, including atypical protein assemblies (like proteopathy) and induced cell death. These similarities suggest that therapeutic advances against one neurodegenerative disease might ameliorate other diseases as well.- neurodegeneration
- neuroinflammation
- apoptosis
- necrosis
- cell death
- oxidative stress
1. Introduction
| Brain Region Affected | Types of Neurodegenerative Diseases |
|---|---|
| Basal ganglia | Parkinson’s disease |
| Huntington disease | |
| Alzheimer’s disease | |
| Frontotemporal degeneration | |
| Thalamus | Alzheimer’s disease |
| Frontotemporal degeneration | |
| Multiple sclerosis | |
| Cerebellum | Multiple sclerosis |
| Multiple systemic atrophy dystonia | |
| Alzheimer’s disease | |
| Spinocerebellar ataxia | |
| Cerebral cortex | Frontotemporal dementia |
| Alzheimer’s disease | |
| Tremors | |
| Parkinson’s disease | |
| Huntington disease | |
| Amyotrophic lateral sclerosis | |
| Neuro psychiatric disorders | |
| Brain stem | Frontotemporal lobar degeneration |
| Parkinson’s disease | |
| Huntington disease | |
| Frontotemporal dementia | |
| Amyotrophic lateral sclerosis | |
| Spinocerebellar ataxia |
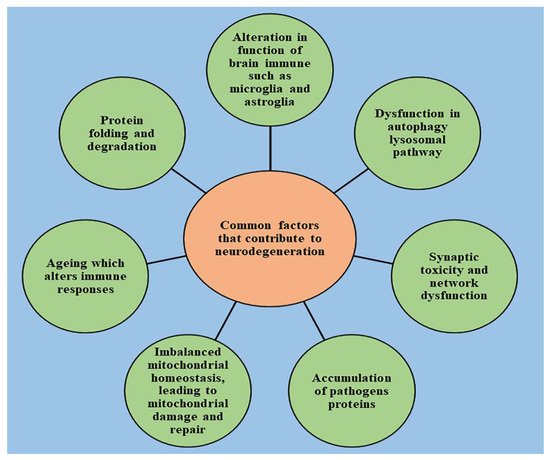
2. Role of Cell Death in the Onset of Neurodegeneration
Neurological disorders are mainly characterized by increased degradation in the functioning of neurons due to the destruction of synapses and axons, eventually leading to nerve cell death. An understanding of the mechanism that leads to the homeostasis of cellular elements and neurodegradation is highly important for developing novel therapeutic treatments for the diseases [15][16][17][18][19]. The healthy cells in the human body transform to preserve the normal homeostasis of tissues; however, post-mitotic neurons harbor very little capacity to regenerate and their survival is essential to ensure the proper functioning of the nervous system [20]. The death of neurons promotes the development of nervous system; however, if occurring in excess, it leads to declined functioning of nervous system and causes the progression of neurodegenerative diseases, which can be indicated by a range of acute insults, from stroke and traumatic brain injury (TBI) to enduring critical conditions such as Parkinson’s disease [21], Alzheimer’s disease [22], and amyotrophic lateral sclerosis [23]. Several studies have been conducted to understand the neuropathology behind the chronic conditions of these diseases, and stereotypical patterns of neurodegeneration have been identified in different regions of the central nervous system, which correspond to the disease severity clinically [24]. Several other pathological pathways, such as impairment in axonal transport and synaptic function, oxidative stress, dysfunction of lysosomes and mitochondria [25][26], activation of microglial cells and protein aggregation, also contribute to neuronal damage [27]. Other factors such as genetics, age, and environmental factors influence the disruption of neuronal homeostasis and aggravate the existing neurodegeneration by activating the signaling of different molecules, ultimately causing cell death and declined functioning of the nervous system [28]. With the advancement of technology in recent years, the understanding of pathology and genetic changes invoked in neurodegenerative diseases has significantly improved but is still unsatisfying. Due to complex biology, the connection between the origin and execution of the death of neurons is still lucid [2]. The pathway involved in cell death and the mechanism responsible for its activation is still under question, and unraveling it is important to drive the development of new target-oriented therapeutic medications. There are several pathways that regulate the cell death of neurons, which are explained below.3. Oxidative Stress and Its Role in Neurodegeneration
The most vital entity for all living organisms is oxygen. Oxygen plays an important role in the physiological functioning of the body, as it is involved in several processes, such as tissue formation, and is a basic element for the growth of every cell. It is highly crucial in inducing gene transcription and signal transduction [29]. However, in excess it may produce detrimental outcomes. The conditions of stress occur mainly due to an imbalance between concentrations of reactive oxygen species (hydroxyl free radical, oxygen) and antioxidants [30]. The imbalance arises mainly due to two factors, which include the excessive synthesis of reactive oxygen species or a disturbance in the antioxidant system of the body [31]. Mitochondria supplies adenosine triphosphate (ATP) to the cells by breaking down glucose molecules, a process called oxidative phosphorylation, and by the synthesis of several other essential biological molecules. The proteins and enzymes required in oxidative phosphorylation are mainly programmed by the DNA of the mitochondria. Apart from the production of ATP via electron transport chain and oxidative phosphorylation, mitochondria are also involved in producing molecules that have a tendency to overcome oxidative stress via apoptotic mechanisms and other functions in cells [32]. The enrichment of mitochondria with enzymes involved in redox reaction and dysfunction of mitochondria is the principal cause of oxidative stress and excessive production of reactive oxygen species in the cellular environment. A phospholipid called cardiolipin is found in the membrane of mitochondria and is specifically involved with proteins in the electron transport chain. Any abnormality in the oxidative phosphorylation process causes the dysfunction of mitochondria cells and the generation of reactive oxygen species (ROS) made up of univalent oxygen molecules. The most important molecule in reactive oxygen species is nitric oxide, which regulates the production and relaxation of the muscle cells of vasculature, leukocyte adhesion, platelet aggregation, angiogenesis, thrombosis, hemodynamics, vascular tone, and many more [33]. The major sources of free radicals include redox metabolism in mitochondria, the metabolism of phospholipids, and several other proteolytic pathways. For normal physiological functioning of the body, the concentration of reactive oxygen species must be low. High concentrations and excessive exposure to ROS leads to the destruction of macromolecules such as DNA, proteins, and lipids, which ultimately cause necrosis and apoptotic cell death [34]. Oxidative stress is also the key element that regulates aging and several other neurological conditions. The chemical integrity of the brain controls the higher functioning of the central nervous system. The human brain is most susceptible to oxidative stress, as it devours a huge proportion of oxygen and is highly rich in lipids [35]. Higher oxygen consumption produces an abundant number of ROS. The membrane of the neurons is made up of polyunsaturated fatty acids, which are also prone to reactive oxygen species [36]. Several neurodegenerative diseases such as Alzheimer’s disease, amyotrophic lateral sclerosis, Huntington disease, and Parkinson’s disease are a result of alterations in biochemical and biomolecular components mainly due to oxidative stress. Hydrogen peroxide, a highly reactive hydroxyl radical and super oxide anion, are some of the negative oxygen species that are involved in neurodegeneration. Therefore, a dire need arises to understand the principal role of oxidative stress in the etiology of neurodegeneration. Apart from reactive oxygen species, reactive nitrogen species such as nitric oxide also possesses destructive effects on neurons [37]. It is important to understand the pathophysiology involved in cell death via ROS and initiate treatment by targeting the specific pathways involved to combat these diseases. Oxidative stress is the predominant factor responsible for the pathogenesis of numerous chronic diseases such as diabetes, rheumatoid arthritis, obesity, etc., and almost all neurological disorders. Scavenging of free radicals by antioxidants can prevent aging and oxidative stress-mediated pathological conditions. The factors that cause oxidative stress and the physiological changes that come as a result of increased reactive oxygen species are presented in Figure 2.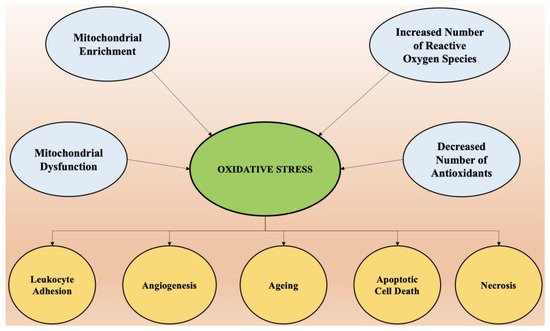
4. Role of Neuroinflammation in the Onset of Neurodegeneration
Recent studies have demonstrated a bridge between chronic inflammation and neurodegeneration. Apoptosis or programmed cell death and necrosis lead to neuronal cell death in the brain [52]. An increased burden of neurodegenerative conditions on the health care system and a lack of effective treatments available pose an urgent need to identify new drug targets. The most common feature that has been found in several neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease is chronic neuroinflammation. Glial cells have been identified as the mediators of neurodegeneration and are responsible for the onset and progression of these diseases. Neuronal health is mainly monitored by the nervous system’s immune cells, called microglia [53]. These cells are activated upon any injury to neurons or infections, which further produces proinflammatory factors (M1 phenotype) or anti-inflammatory factors (M2 phenotype). For a healthy functioning of the human brain, it is necessary to have the correct balance between anti-inflammatory mediators, which allow for the repair and healing of tissues, and proinflammatory mediators, which clear the cellular debris and misfolded protein aggregation is maintained [54]. The activation of microglia cells in Alzheimer’s and Parkinson’s disease is mainly tilted towards the M1 phenotype, which leads to an exaggeration of inflammation and accelerates the progression of the disease [55]. The new therapies for the management of neurodegeneration include induction of the M2 phenotype and deactivation of the M1 phenotype in the brain. Astrocytes are another type of glial cells that are present in the brain and regulate the maintenance and maturation of neuronal cells. They are highly sensitive and respond to injury very quickly, as do microglia cells [56]. Depending upon their activation, they can be neuroprotective by stimulating repair and reducing inflammation or be neurotoxic by promoting inflammation and contributing to the death of neuronal cells. Activated astrocytes also act as a proinflammatory factor in Parkinson’s disease and Alzheimer’s disease [57]. Their role has been implicated in the breakdown of the blood–brain barrier, thereby encouraging the infiltration of immune cells into the brain, which increases neuronal death by excessive stimulation and impairment of the uptake of neurotransmitter glutamate. Another type of glial cell, called oligodendrocytes, also poses a significant role in neurodegeneration [58]. Oligodendrocytes form a sheath of myelin around the nerve fibers, which permits the efficient and rapid transmission of electrical impulses across neurons and thus induces signal transmission. Damage in these cells has been attributed to the progression of multiple sclerosis and other neurodegenerative diseases in which the immune system attacks oligodendrocytes and damages the myelin sheath, thereby reducing levels of myelin proteins in brain tissue [59].5. Conclusions
1. Specific Disorders
Alzheimer's disease
Alzheimer's disease (AD) is a chronic neurodegenerative disease that results in the loss of neurons and synapses in the cerebral cortex and certain subcortical structures, resulting in gross atrophy of the temporal lobe, parietal lobe, and parts of the frontal cortex and cingulate gyrus.[5] It is the most common neurodegenerative disease.[1] Even with billions of dollars being used to find a treatment for Alzheimer's disease, no effective treatments have been found.[6] However, clinical trials have developed certain compounds that could potentially change the future of Alzheimer's disease treatments.[7] Currently, diagnoses of Alzheimer's is subpar, and better methods need to be utilized for various aspects of clinical diagnoses.[8] Alzheimer's has a 20% misdiagnosis rate.[8] AD pathology is primarily characterized by the presence of senile plaques and neurofibrillary tangles. Plaques are made up of small peptides, typically 39–43 amino acids in length, called beta-amyloid (also written as A-beta or Aβ). Beta-amyloid is a fragment from a larger protein called amyloid precursor protein (APP), a transmembrane protein that penetrates through the neuron's membrane. APP appears to play roles in normal neuron growth, survival and post-injury repair.[9][10] APP is cleaved into smaller fragments by enzymes such as gamma secretase and beta secretase.[11] One of these fragments gives rise to fibrils of beta-amyloid which can self-assemble into the dense extracellular deposits known as senile plaques or amyloid plaques.[12][13]
Parkinson's disease
Parkinson's disease (PD) is the second most common neurodegenerative disorder.[14] It typically manifests as bradykinesia, rigidity, resting tremor and posture instability. The crude prevalence rate of PD has been reported to range from 15 per 100,000 to 12,500 per 100,000, and the incidence of PD from 15 per 100,000 to 328 per 100,000, with the disease being less common in Asian countries. PD is primarily characterized by death of dopaminergic neurons in the substantia nigra, a region of the midbrain. The cause of this selective cell death is unknown. Notably, alpha-synuclein-ubiquitin complexes and aggregates are observed to accumulate in Lewy bodies within affected neurons. It is thought that defects in protein transport machinery and regulation, such as RAB1, may play a role in this disease mechanism.[15] Impaired axonal transport of alpha-synuclein may also lead to its accumulation in Lewy bodies. Experiments have revealed reduced transport rates of both wild-type and two familial Parkinson's disease-associated mutant alpha-synucleins through axons of cultured neurons.[16] Membrane damage by alpha-synuclein could be another Parkinson's disease mechanism.[17] The main known risk factor is age. Mutations in genes such as α-synuclein (SNCA), leucine-rich repeat kinase 2 (LRRK2), glucocerebrosidase (GBA), and tau protein (MAPT) can also cause hereditary PD or increase PD risk.[18] While PD is the second most common neurodegenerative disorder, problems with diagnoses still persist.[19] Problems with the sense of smell is a widespread symptom of Parkinson’s disease (PD), however, some neurologists question its efficacy.[19] This assessment method is a source of controversy among medical professionals.[19] The gut microbiome might play a role in the diagnosis of PD, and research suggests various ways that could revolutionize the future of PD treatment.[20]
Huntington's disease
Huntington's disease (HD) is a rare autosomal dominant neurodegenerative disorder caused by mutations in the huntingtin gene (HTT). HD is characterized by loss of medium spiny neurons and astrogliosis.[21][22][23] The first brain region to be substantially affected is the striatum, followed by degeneration of the frontal and temporal cortices.[24] The striatum's subthalamic nuclei send control signals to the globus pallidus, which initiates and modulates motion. The weaker signals from subthalamic nuclei thus cause reduced initiation and modulation of movement, resulting in the characteristic movements of the disorder, notably chorea.[25] Huntington's disease presents itself later in life even though the proteins that cause the disease works towards manifestation from their early stages in the humans affected by the proteins.[26] Along with being a neurodegenerative disorder, HD has links to problems with neurodevelopment.[26] HD is caused by polyglutamine tract expansion in the huntingtin gene, resulting in the mutant huntingtin. Aggregates of mutant huntingtin form as inclusion bodies in neurons, and may be directly toxic. Additionally, they may damage molecular motors and microtubules to interfere with normal axonal transport, leading to impaired transport of important cargoes such as BDNF.[16] Huntington's disease currently has no effective treatments that would modify the disease.[27]
Multiple sclerosis (MS)
Multiple sclerosis is a chronic debilitating demyelinating disease of the central nervous system, caused by an autoimmune attack resulting in the progressive loss of myelin sheath on neuronal axons.[28] The resultant decrease in the speed of signal transduction leads to a loss of functionality that includes both cognitive and motor impairment depending on the location of the lesion.[28] The progression of MS occurs due to episodes of increasing inflammation, which is proposed to be due to the release of antigens such as myelin oligodendrocyte glycoprotein, myelin basic protein, and proteolipid protein, causing an autoimmune response.[29] This sets off a cascade of signaling molecules that result in T cells, B cells, and Macrophages to cross the blood-brain barrier and attack myelin on neuronal axons leading to inflammation.[30] Further release of antigens drives subsequent degeneration causing increased inflammation.[31] Multiple sclerosis presents itself as a spectrum based on the degree of inflammation, a majority of patients suffer from early relapsing and remitting episodes of neuronal deterioration following a period of recovery. Some of these individuals may transition to a more linear progression of the disease, while about 15% of others begin with a progressive course on the onset of Multiple sclerosis. The inflammatory response contributes to the loss of the grey matter, and as a result current literature devotes itself to combatting the auto-inflammatory aspect of the disease.[30] While there are several proposed causal links between EBV and the HLA-DRB1*15:01 allele to the onset of Multiple Sclerosis they may contribute to the degree of autoimmune attack and the resultant inflammation, they do not determine the onset of Multiple Sclerosis.[30]
Amyotrophic lateral sclerosis (ALS)
Amyotrophic lateral sclerosis (ALS or Lou Gehrig's disease) is a disease in which motor neurons are selectively targeted for degeneration. Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder that negatively impacts the upper motor neurons (UMNs) and lower motor neurons (LMNs).[32] In 1993, missense mutations in the gene encoding the antioxidant enzyme Cu/Zn superoxide dismutase 1 (SOD1) were discovered in a subsets of patients with familial ALS. This discovery led researchers to focus on unlocking the mechanisms for SOD1-mediated diseases. However, the pathogenic mechanism underlying SOD1 mutant toxicity has yet to be resolved. More recently, TDP-43 and FUS protein aggregates have been implicated in some cases of the disease, and a mutation in chromosome 9 (C9orf72) is thought to be the most common known cause of sporadic ALS. It is diagnosed by skeletal muscle weakness that progresses gradually.[32] Early diagnosis of ALS is harder than with other neurodegenerative diseases as there are no highly effective means of determining its early onset.[32] Currently, there is research being done regarding the diagnosis of ALS through upper motor neuron tests.[33] The Penn Upper Motor Neuron Score (PUMNS) consists of 28 criteria with a score range of 0-32.[33] A higher score indicates a higher level of burden present on the upper motor neurons.[33] The PUMNS has proven quite effective in determining the burden that exists on upper motor neurons in affected patients.[33] Independent research provided in vitro evidence that the primary cellular sites where SOD1 mutations act are located on astrocytes.[34][35] Astrocytes then cause the toxic effects on the motor neurons. The specific mechanism of toxicity still needs to be investigated, but the findings are significant because they implicate cells other than neuron cells in neurodegeneration.[36]
Batten disease
Batten disease is a rare and fatal recessive neurodegenerative disorder that begins in childhood.[37] Batten disease is the common name for a group of lysosomal storage disorders known as neuronal ceroid lipofuscinoses (NCLs) – each caused by a specific gene mutation,[37] of which there are thirteen.[38] Since Batten disease is quite rare, its worldwide prevalence is about 1 in every 100,000 live births.[39] In North America, CLN3 disease (juvenile NCL) typically manifests between the ages of 4 to 7.[40] Batten disease is characterized by motor impairment, epilepsy, dementia, vision loss, and shortened lifespan.[41] A loss of vision is common first sign of Batten disease.[40] Loss of vision is typically preceded by cognitive and behavioral changes, seizures, and loss of the ability to walk.[40] It is common for people to establish cardiac arrhythmias and difficulties eating food as the disease progresses.[40] Batten disease diagnosis depends on a conflation of many criteria: clinical signs and symptoms, evaluations of the eye, electroencephalograms (EEG), and brain magnetic resonance imaging (MRI) results.[39] The diagnosis provided by these results are corroborated by genetic and biochemical testing.[39] No effective treatments were available to prevent the disease from being widespread before the past few years.[39] In recent years, more models have been created to expedite the research process for methods to treat Batten disease.[39]
2. Risk Factor
The greatest risk factor for neurodegenerative diseases is aging. Mitochondrial DNA mutations as well as oxidative stress both contribute to aging.[42] Many of these diseases are late-onset, meaning there is some factor that changes as a person ages for each disease.[3] One constant factor is that in each disease, neurons gradually lose function as the disease progresses with age. It has been proposed that DNA damage accumulation provides the underlying causative link between aging and neurodegenerative disease.[43][44] About 20-40% of healthy people between 60 and 78 years old experience discernable decrements in cognitive performance in several domains including working, spatial, and episodic memory, and processing speed.[45]
3. Mechanisms
Genetics
Many neurodegenerative diseases are caused by genetic mutations, most of which are located in completely unrelated genes. In many of the different diseases, the mutated gene has a common feature: a repeat of the CAG nucleotide triplet. CAG codes for the amino acid glutamine. A repeat of CAG results in a polyglutamine (polyQ) tract. Diseases associated with such mutations are known as trinucleotide repeat disorders.[46][47] Polyglutamine repeats typically cause dominant pathogenesis. Extra glutamine residues can acquire toxic properties through a variety of ways, including irregular protein folding and degradation pathways, altered subcellular localization, and abnormal interactions with other cellular proteins.[46] PolyQ studies often use a variety of animal models because there is such a clearly defined trigger – repeat expansion. Extensive research has been done using the models of nematode (C. elegans), and fruit fly (Drosophila), mice, and non-human primates.[47][48] Nine inherited neurodegenerative diseases are caused by the expansion of the CAG trinucleotide and polyQ tract, including Huntington's disease and the spinocerebellar ataxias.[49]
Protein misfolding
Several neurodegenerative diseases are classified as proteopathies as they are associated with the aggregation of misfolded proteins. Protein toxicity is one of the key mechanisms of many neurodegenrative diseases.[50]
- alpha-synuclein: can aggregate to form insoluble fibrils in pathological conditions characterized by Lewy bodies, such as Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. Alpha-synuclein is the primary structural component of Lewy body fibrils. In addition, an alpha-synuclein fragment, known as the non-Abeta component (NAC), is found in amyloid plaques in Alzheimer's disease.
- tau: hyperphosphorylated tau protein is the main component of neurofibrillary tangles in Alzheimer's disease; tau fibrils are the main component of Pick bodies found in behavioral variant frontotemporal dementia.
- beta amyloid: the major component of senile plaques in Alzheimer's disease.
- prion: main component of prion diseases and transmissible spongiform encephalopathy.
Intracellular mechanisms
Protein degradation pathways
Parkinson's disease and Huntington's disease are both late-onset and associated with the accumulation of intracellular toxic proteins. Diseases caused by the aggregation of proteins are known as proteinopathies, and they are primarily caused by aggregates in the following structures:[3]
- cytosol, e.g. Parkinson's & Huntington's
- nucleus, e.g. Spinocerebellar ataxia type 1 (SCA1)
- endoplasmic reticulum (ER), (as seen with neuroserpin mutations that cause familial encephalopathy with neuroserpin inclusion bodies)
- extracellularly excreted proteins, amyloid-β in Alzheimer's disease
There are two main avenues eukaryotic cells use to remove troublesome proteins or organelles:
- ubiquitin–proteasome: protein ubiquitin along with enzymes is key for the degradation of many proteins that cause proteinopathies including polyQ expansions and alpha-synucleins. Research indicates proteasome enzymes may not be able to correctly cleave these irregular proteins, which could possibly result in a more toxic species. This is the primary route cells use to degrade proteins.[3]
- Decreased proteasome activity is consistent with models in which intracellular protein aggregates form. It is still unknown whether or not these aggregates are a cause or a result of neurodegeneration.[3]
- autophagy–lysosome pathways: a form of programmed cell death (PCD), this becomes the favorable route when a protein is aggregate-prone meaning it is a poor proteasome substrate. This can be split into two forms of autophagy: macroautophagy and chaperone-mediated autophagy (CMA).[3]
- macroautophagy is involved with nutrient recycling of macromolecules under conditions of starvation, certain apoptotic pathways, and if absent, leads to the formation of ubiquinated inclusions. Experiments in mice with neuronally confined macroautophagy-gene knockouts develop intraneuronal aggregates leading to neurodegeneration.[3]
- chaperone-mediated autophagy defects may also lead to neurodegeneration. Research has shown that mutant proteins bind to the CMA-pathway receptors on lysosomal membrane and in doing so block their own degradation as well as the degradation of other substrates.[3]
Membrane damage
Damage to the membranes of organelles by monomeric or oligomeric proteins could also contribute to these diseases. Alpha-synuclein can damage membranes by inducing membrane curvature,[17] and cause extensive tubulation and vesiculation when incubated with artificial phospholipid vesicles.[17] The tubes formed from these lipid vesicles consist of both micellar as well as bilayer tubes. Extensive induction of membrane curvature is deleterious to the cell and would eventually lead to cell death.Apart from tubular structures, alpha-synuclein can also form lipoprotein nanoparticles similar to apolipoproteins.
Mitochondrial dysfunction
The most common form of cell death in neurodegeneration is through the intrinsic mitochondrial apoptotic pathway. This pathway controls the activation of caspase-9 by regulating the release of cytochrome c from the mitochondrial intermembrane space. Reactive oxygen species (ROS) are normal byproducts of mitochondrial respiratory chain activity. ROS concentration is mediated by mitochondrial antioxidants such as manganese superoxide dismutase (SOD2) and glutathione peroxidase. Over production of ROS (oxidative stress) is a central feature of all neurodegenerative disorders. In addition to the generation of ROS, mitochondria are also involved with life-sustaining functions including calcium homeostasis, PCD, mitochondrial fission and fusion, lipid concentration of the mitochondrial membranes, and the mitochondrial permeability transition. Mitochondrial disease leading to neurodegeneration is likely, at least on some level, to involve all of these functions.[51] There is strong evidence that mitochondrial dysfunction and oxidative stress play a causal role in neurodegenerative disease pathogenesis, including in four of the more well known diseases Alzheimer's, Parkinson's, Huntington's, and Amyotrophic lateral sclerosis.[42] Neurons are particularly vulnerable to oxidative damage due to their strong metabolic activity associated with high transcription levels, high oxygen consumption, and weak antioxidant defense.[52][53]
DNA damage
The brain metabolizes as much as a fifth of consumed oxygen, and reactive oxygen species produced by oxidative metabolism are a major source of DNA damage in the brain. Damage to a cell’s DNA is particularly harmful because DNA is the blueprint for protein production and unlike other molecules it cannot simply be replaced by re-synthesis. The vulnerability of post-mitotic neurons to DNA damage (such as oxidative lesions or certain types of DNA strand breaks), coupled with a gradual decline in the activities of repair mechanisms, could lead to accumulation of DNA damage with age and contribute to brain aging and neurodegeneration.[54] DNA single-strand breaks are common and are associated with the neurodegenerative disease ataxia-oculomotor apraxia.[55][53] Increased oxidative DNA damage in the brain is associated with Alzheimer’s disease and Parkinson’s disease.[55] Defective DNA repair has been linked to neurodegenerative disorders such as Alzheimer’s disease, amyotrophic lateral sclerosis, ataxia telangiectasia, Cockayne syndrome, Parkinson’s disease and xeroderma pigmentosum.[55][54]
Axonal transport
Axonal swelling, and axonal spheroids have been observed in many different neurodegenerative diseases. This suggests that defective axons are not only present in diseased neurons, but also that they may cause certain pathological insult due to accumulation of organelles. Axonal transport can be disrupted by a variety of mechanisms including damage to: kinesin and cytoplasmic dynein, microtubules, cargoes, and mitochondria.[16] When axonal transport is severely disrupted a degenerative pathway known as Wallerian-like degeneration is often triggered.[56]
Programmed cell death
Programmed cell death (PCD) is death of a cell in any form, mediated by an intracellular program.[57] This process can be activated in neurodegenerative diseases including Parkinson's disease, amytrophic lateral sclerosis, Alzheimer's disease and Huntington's disease.[58] PCD observed in neurodegenerative diseases may be directly pathogenic; alternatively, PCD may occur in response to other injury or disease processes.[4]
Apoptosis (type I)
Apoptosis is a form of programmed cell death in multicellular organisms. It is one of the main types of programmed cell death (PCD) and involves a series of biochemical events leading to a characteristic cell morphology and death.
- Extrinsic apoptotic pathways: Occur when factors outside the cell activate cell surface death receptors (e.g., Fas) that result in the activation of caspases-8 or -10.[4]
- Intrinsic apoptotic pathways: Result from mitochondrial release of cytochrome c or endoplasmic reticulum malfunctions, each leading to the activation of caspase-9. The nucleus and Golgi apparatus are other organelles that have damage sensors, which can lead the cells down apoptotic pathways.[4][59]
Caspases (cysteine-aspartic acid proteases) cleave at very specific amino acid residues. There are two types of caspases: initiators and effectors. Initiator caspases cleave inactive forms of effector caspases. This activates the effectors that in turn cleave other proteins resulting in apoptotic initiation.[4]
Autophagic (type II)
Autophagy is a form of intracellular phagocytosis in which a cell actively consumes damaged organelles or misfolded proteins by encapsulating them into an autophagosome, which fuses with a lysosome to destroy the contents of the autophagosome. Because many neurodegenerative diseases show unusual protein aggregates, it is hypothesized that defects in autophagy could be a common mechanism of neurodegeneration.[4]
Cytoplasmic (type III)
PCD can also occur via non-apoptotic processes, also known as Type III or cytoplasmic cell death. For example, type III PCD might be caused by trophotoxicity, or hyperactivation of trophic factor receptors. Cytotoxins that induce PCD can cause necrosis at low concentrations, or aponecrosis (combination of apoptosis and necrosis) at higher concentrations. It is still unclear exactly what combination of apoptosis, non-apoptosis, and necrosis causes different kinds of aponecrosis.[4]
Transglutaminase
Transglutaminases are human enzymes ubiquitously present in the human body and in the brain in particular.[60] The main function of transglutaminases is bind proteins and peptides intra- and intermolecularly, by a type of covalent bonds termed isopeptide bonds, in a reaction termed transamidation or crosslinking.[60] Transglutaminase binding of these proteins and peptides make them clump together. The resulting structures are turned extremely resistant to chemical and mechanical disruption.[60] Most relevant human neurodegenerative diseases share the property of having abnormal structures made up of proteins and peptides.[60] Each of these neurodegenerative diseases have one (or several) specific main protein or peptide. In Alzheimer's disease, these are amyloid-beta and tau. In Parkinson’s disease, it is alpha-synuclein. In Huntington’s disease, it is huntingtin.[60] Transglutaminase substrates: Amyloid-beta, tau, alpha-synuclein and huntingtin have been proved to be substrates of transglutaminases in vitro or in vivo, that is, they can be bonded by trasglutaminases by covalent bonds to each other and potentially to any other transglutaminase substrate in the brain.[60] Transglutaminase augmented expression: It has been proved that in these neurodegenerative diseases (Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease) the expression of the transglutaminase enzyme is increased.[60] Presence of isopeptide bonds in these structures: The presence of isopeptide bonds (the result of the transglutaminase reaction) have been detected in the abnormal structures that are characteristic of these neurodegenerative diseases.[60] Co-localization: Co-localization of transglutaminase mediated isopeptide bonds with these abnormal structures has been detected in the autopsy of brains of patients with these diseases.[60]
4. Management
The process of neurodegeneration is not well understood, so the diseases that stem from it have, as yet, no cures.
Animal models in research
In the search for effective treatments (as opposed to palliative care), investigators employ animal models of disease to test potential therapeutic agents. Model organisms provide an inexpensive and relatively quick means to perform two main functions: target identification and target validation. Together, these help show the value of any specific therapeutic strategies and drugs when attempting to ameliorate disease severity. An example is the drug Dimebon by Medivation, Inc. In 2009 this drug was in phase III clinical trials for use in Alzheimer's disease, and also phase II clinical trials for use in Huntington's disease.[47] In March 2010, the results of a clinical trial phase III were released; the investigational Alzheimer's disease drug Dimebon failed in the pivotal CONNECTION trial of patients with mild-to-moderate disease.[61] With CONCERT, the remaining Pfizer and Medivation Phase III trial for Dimebon (latrepirdine) in Alzheimer's disease failed in 2012, effectively ending the development in this indication.[62] In another experiment using a rat model of Alzheimer's disease, it was demonstrated that systemic administration of hypothalamic proline-rich peptide (PRP)-1 offers neuroprotective effects and can prevent neurodegeneration in hippocampus amyloid-beta 25–35. This suggests that there could be therapeutic value to PRP-1.[63]
Other avenues of investigation
Protein degradation offers therapeutic options both in preventing the synthesis and degradation of irregular proteins. There is also interest in upregulating autophagy to help clear protein aggregates implicated in neurodegeneration. Both of these options involve very complex pathways that we are only beginning to understand.[3] The goal of immunotherapy is to enhance aspects of the immune system. Both active and passive vaccinations have been proposed for Alzheimer's disease and other conditions; however, more research must be done to prove safety and efficacy in humans.[64] A current therapeutic target for the treatment of Alzheimer's disease is the protease β-secretase[65], which is involved in the amyloidogenic processing pathway that leads to the pathological accumulation of proteins in the brain. When the gene that encodes for amyloid precursor protein (APP) is spliced by α-secretase[66] rather than β-secretase, the toxic protein β amyloid is not produced. Targeted inhibition[67] of β-secretase can potentially prevent the neuronal death that is responsible for the symptoms of Alzheimer's disease.

