Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Nesrein Hashem and Version 2 by Dean Liu.
Nowadays, smart ART that considers technique efficiency, animal welfare, cost efficiency and environmental health are developed. Recently, the nanotechnology revolution has pervaded all scientific fields including the reproduction of farm animals, facilitating certain improvements in this field. Nanotechnology could be used to improve and overcome many technical obstacles that face different ART.
- hormone
- nanotechnology
- pregnancy
- reproduction
- semen
1. Introduction
Reproductive management, and specifically the use of assisted reproductive technologies (ART), is a fast-growing field, which is essential for the manipulation of reproduction in the livestock sector. The main aim is to develop and implement relevant tools for improving fertility of farm animals with easily applied, affordable and effective techniques. Currently, such philosophy has to take into account the concept of smart production, which incorporates not only concepts as technique-efficiency and cost-efficiency, but also animal welfare, human health and environmental safety [1][2]. Therefore, great efforts are made by scientists to achieve the aims of smart production, particularly in the field of animal reproduction and specifically in the management of the reproductive activity, artificial insemination (AI), multiple ovulation and embryo transfer (MOET) and the management of pregnancy. These areas make necessary the administration of hormones, growth-factors and other substances and the manipulation of living cells like spermatozoa, oocytes and embryos. The efficiency of these processes is compromised by technical and economic issues, but also by environmental and health concerns, and all these aspects may be improved by the use of nanotechnology.
Nanotechnology is an innovative discipline, combining the sciences of physics, chemistry, biology, mathematics as well as engineering and computer sciences. Nanotechnology has been used for the development of diagnostic and therapeutic agents in human medicine, but its application in animal medicine and production is still scarce. The concept of this technology is to transform chemical molecules into small-scale size particles (range 1–200 nm). Consequently, new physical and chemical properties are achieved, including greater cellular uptake, reactivity, surface area and charge, and binding properties which might have opened a new platform for biosciences innovation [2][3][3,4]. Nano-sized drug delivery systems (Figure 1) are being developed, both as therapeutic agents and as therapy delivery systems, because of the use of germicidal properties of metal and polymeric nanoparticles as antimicrobials, while natural and nanostructured materials can entrap and protect compounds for delivery [4][5]. Many nanomaterials may be useful to overcome some obstacles that compromise the efficiency of ART in animal production.
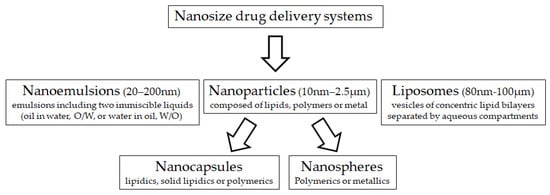
Figure 1. Main nano-sized material used for drug delivery systems.
Specifically, for the male, at post-collection semen handling for AI, specific nanomaterials are being designed to obtain semen doses with high sperm quality, by optimizing both semen purification (removing of defective sperm cells) and semen preservation (cooling or freezing) processes. For example, nano-magnetic pods of iron oxide (Fe3O4 NPs), characterized by their bio-compatibility and bio-function [5][6], have been used to purify both fresh semen ejaculates and frozen-thawed semen previously to be applied in the field scale AI or in vitro fertilization (IVF), respectively. The first studies indicate the ability of producing semen doses with high recovered purified sperm cells in boars [6][7] and increased conception rates following AI in cattle [7][8]. Nanoparticles of compounds with high antioxidant activity such as cerium oxide [8][9], selenium [9][10] and zinc [10][11] can be also used as semen extender additives, to protect frozen semen from the negative effects of reactive oxygen species during the cryopreservation process.
2. Nanotechnologies for Management of Cycle and Implementation of ART in Females
In this section, the advantages of nano-drug delivery technologies for smart management of the reproductive cycle in females will be discussed, with particular focus on the mechanisms involved. In conventional drug delivery systems, the bioavailability of the drug relies on several factors, such as the permeability throughout the epithelial and endothelial cells, the solubility, the passage through blood barriers, the clearance rate by liver and kidney and the resistance against systemic and circulating degradation enzymes. Thus, smart nanostructured delivery systems, particularly those depending on the polymerization of bioactive molecules using biodegradable and biocompatible polymers, are engineered with different physicochemical properties acting via different mechanisms, to efficiently deliver drugs from the site of administration to the sites of action, overcoming the unfavorable drug properties and the biological barriers [11][30]. One of the mechanisms by which NP-based drug delivery systems can protect drugs from enzymatic degradation and rapid clearance depends on the ability of the nanoformula to absorb and/or encapsulate the drugs. The other mechanisms by which NP-based drug delivery systems can improve drug pharmacokinetic and pharmacodynamic properties are related to the physicochemical properties of the nanoformula such as shape, particle size, surface charge (zeta potential) and particles polydispersity (PdI). Particle size can play significant roles in the determination of cellular uptake and degradation and elimination processes of the drug. Small sized-Nps can escape from reticuloendothelial and mononuclear phagocytic systems, leading to an increase in total blood circulation time and bioavailability. Additionally, surface charge characteristics can affect the degree of NPs uptake according to the charge of tissue cells. Many cellular membranes are negatively charged; thus, cationic NPs facilitate membrane attraction and adhesion, which create favorable properties for cellular uptake, via endocytosis or other mechanisms [12][31]. Based on the previously mentioned scientific facts, many drugs used for managing reproductive processes in females were developed to have new properties meeting the smart reproductive management aims.2.1. Nano-Hormone Delivery Systems and Cycle Management
Procedures for implementing ART in farm animals are based on the administration of exogenous reproductive hormones (mainly gonadotrophins, steroids and prostaglandins) for controlling the reproductive cycle, improving reproductive efficiency and treating some reproductive disorders [13][14][15][12,32,33]. However, the effectiveness of hormonal treatments for controlling farm animals’ reproduction is limited by several factors [1][16][17][2,14,34]: 1. the biological activity of the hormone (which depends on its bioavailability, kinetics and dose) 2. the animal response to the hormonal treatment after repeated treatments (some hormones evoke the formation of antibodies); 3. the cost of the applied protocol; 4. animal health and welfare; 5. environmental-related issues (release of xenobiotics and also waste to the environment in the case of undegradable hormone carriers); and 6. consumers’ health (raising concern about residual effect in tissues and thus the possibility of consumption of animal products with hormonal residues).
The use of nano-drug, or nano-hormone delivery systems (NHDS) may be useful for alleviating the incidence of these factors. Firstly, enhancing the biological efficiency of the hormonal treatment; conjugation of the hormone with a suitable nano-particle is hypothesized to increase its half-life, improve its passage across epithelial or endothelial barriers into blood or lymph circulation and sustain its delivery to the target sites, improving their uptake by cells [18][35]; hence, it is possible to use lower doses. As an example, gonadotrophin releasing hormone (GnRH) is included in some prostaglandin-based protocols, since it induces ovulation and has luteotrophic effects favoring progesterone secretion and the maintenance of pregnancy [19][36]. However, GnRH has a short half-life time in blood circulation, which diminishes both its biological activity and sustained action. Hashem and Sallam [3][4] found that the administration of chitosan- sodium tripolyphosphate (TPP)-conjugated GnRH nanoparticles in goats allowed 75% reduction in the GnRH dose, without affecting fertility and prolificacy, which supports that the nanoformula increases the bioavailability of the hormone. In this study, the main proposed mechanism involved in the reduction of the GnRH dose was the ability of nanoformula to increase the bioavailability of the GnRH to the brain (pituitary, target organ) as the size, PdI and zeta potential of fabricated chitosan-TPP-conjugated gonadorelin NPs were 93.91nm, 0.302 and 11.6 mV, respectively, with 91.2% entrapment efficiency for GnRH. NPs of a size ranging from 50 to 200 nm, a PdI less than or equal to 0.3 and a low positive charge (up to 15 mV) are efficient for drug delivery to the brain [20][37].
In addition to allowing the use of a decreased dose, NHDS may also be used to change the route of administration [19][36] and therefore increase animal welfare and decrease the risk of exposition to different hormones by workers and technicians [21][38]. Specifically, protocols for AI in rabbits usually include GnRH-supplemented extenders to induce ovulation via semen dose. This is certainly a good example of a welfare-orientated method, being a non-invasive route which diminishes animal distress and, labor amount and working time when compared to conventional treatments via intramuscular doses. However, the biological barriers for mucosal permeation and the proteolytic activity of seminal plasma and vaginal fluids limit this technique and, to achieve fertility results similar to the conventional intramuscular protocol, the hormone concentration administered intravaginally should be about ten-folds higher, which constitutes a potential health risk for workers. As an attempt to alleviate these risks, chitosan–dextran sulphate GnRH (buserelin acetate) NPs have been fabricated and supplemented to semen extender of rabbits, which allows a 50% of reduction in the dose without affecting fertility [16][14]. Similar results were obtained by Hassanein et al. [22][39] when chitosan-TPP GnRH NPs were used to induce ovulation by applying the chitosan-TPP GnRH NPs intramuscularly or intravaginally during AI process. These results were attributed to the protective role of chitosan and dextran sulfate NPs for GnRH against the negative action of vaginal proteolytic enzymes, and the improved mucoadhesive properties of the nanoformula [16][14]. Cationic charged polymers such as chitosan yield an attractive force with the anionic polyelectrolyte properties of vaginal mucus, resulting in enhanced muco-adhesion and the retention of NPs within the mucus layer, allowing both sustained release and higher cellular uptake by vaginal mucosa [12][31].
Finally, conventional hormonal treatments are also upsetting environmental issues, due to the release of hormonal residues and carrier materials into the environment. The most evident example is the use of progesterone-impregnated intravaginal devices (e.g.,: CIDR, PRID and Cue-Mate) which were developed for cattle, and afterwards for small ruminants, since the 1970s and which are currently available worldwide [23][24][40,41]. The main matrix of these devices is composed of silicon polymers, which need to be loaded with high progesterone concentrations to release enough hormones to the vaginal mucosa. Progesterone levels into the device remain high after discard and currently, inserts based on polyethylene vinyl acetate (EVA) copolymers polyisoprene and polymethyl-methacrylate are being developed for reducing progesterone charge, thus diminishing the costs and emissions of hormones to the environment [1][25][2,42]. However, similarly to silicone-based devices, these materials are not biodegradable. In view of these circumstances, biodegradable and biocompatible polymeric materials, such as chitosan and poly (lactic acid) (PLA), poly (glycolic acid) (PGA) and PLGA polymers are being tested.
In view of these circumstances, Helbling and coworkers [26][43] developed a complex of chitosan-tpp-Tween80 as a biodegradable carrier for progesterone using the spray-drying technique. The particles obtained by this method were at micro size (ranged between 1 and 7 μm) and 69%–75% encapsulation efficiency; however, the authors supported its efficacy as a biodegradable delivery system for progesterone in cattle. Oliveira and coworkers [27][15] developed and tested biodegradable and biocompatible nanomaterials (specifically nanofibrous mats of polylactic acid; PLA) loaded with progesterone by solution blow spinning technique, with promising results for controlled progesterone delivery. Similarly, Fogolari and coworkers [25][42] used a method of miniemulsion polymerization for producing two forms of progesterone-conjugated nanocapsules: nanospheres (NS) and nanocapsules (NC), using polymethyl-methacrylate, a biocompatible polymer, instead of silicone-based release devices. The encapsulation efficiencies of NS and NC were greater than 69% and 90%, with average sizes of 150–200 nm and 240–300 nm, respectively, which supports the usefulness of this method for progesterone binding.
However, the few studies performed in this respect have shown promising results regarding the replacement of non-biodegradable progesterone carriers with biodegradable ones using advances of NHDS; the application of such technology on farm scale needs more efforts to be justified [26][43]. This is simply because the use of vaginal drug nanocarriers has to consider many biological mechanisms, including the protection of labile molecules from adverse environmental agents, controlled release, the modulation of adhesion to mucus, mucosal tissue penetration, specific targeting, and intracellular delivery.
2.2. Nano-Drug Delivery Systems and Management of Pregnancy
Pregnancy and delivery are pivotal facts for the reproductive management and productivity of farm animals. One of the main complications of pregnancy in most farm animals, as in other many species including humans, is the occurrence of intrauterine growth restriction (IUGR) [28][29][30][45,46,47]. IUGR is the failure of a fetus to reach its full genetic growth potential [31][48]) and its appearance may be related to genetic traits, infections or, more frequently, to an intrauterine environment inadequate for the development of the fetus. The main factor affecting the intrauterine environment is the availability of nutrients and oxygen required by the fetus for its development, so maternal undernutrition, heat stress and/or hypoxia are the main causes of IUGR. The placenta is the organ driving the transfer of nutrients and oxygen from the mother and the fetus, so alterations in placental development (by the crowding of uterine space in case of twins in ruminants or hyperprolificacy in pigs and rabbits) and placental efficiency (by inadequate function) are also main causes for IUGR.
The main therapies for IUGR, in the case of farm animals, are based on maternal nutritional supplementation with amino acids favoring placental and fetal neoangiogenesis and tissue development, with vitamins favoring protein synthesis and antioxidant capacity and with other antioxidant agents like polyphenols [12][31]. In humans, pharmacological treatments including the use of aspirin, sidenafil citrate or metformin are also applied. However, all these therapies are based on actions at the systemic level, which makes the administration of these substances at high doses to the mother to allow their availability at the fetoplacental level necessary, with the obvious risk to mother and fetus. Future prospects are based on focused treatments, either directly at the fetus, or by using infusion of umbilical cord, amniotic sac or placenta, with lower risk.
In this field, the infusion of the therapies throughout the umbilical cord, the amniotic sac or the placenta may be clearly optimized by the used nanoparticles. Nanoparticles can be directly used as a tool for the targeted delivery of therapeutics to the placenta. In this sense, surface-functionalized nanoparticles focused on receptors exclusively expressed at the placenta are giving promising results for their future use in the targeted therapy of pregnancy complications. In this sense, we have to highlight the works using plCSA binding peptide (plCSA-BP) nanoparticles [32][49], which target the placenta via placental chondroitin sulfate A (plCSA). These strategies would enable one not only to direct the drug to the fetus, but also to increase the effectiveness and specificity of the carried compound and to decrease collateral effects [33][50]. Prospective studies for possible treatments with nanoparticles have been based mainly on the administration of growth factors and nitric oxide donors [34][51].
On the other hand, pregnancy complications may not directly affect the fetus but indirectly, through negative effects on the the maternal side, the uterus and maternal placenta. In this case, the objective would be to treat the mother without affecting the fetus. A classic example is preterm labor, which needs to be treated with tocolytic drugs like indomethacin which freely crosses the placenta and seriously affects the fetus (narrowing the ductus arteriosus and causing hydramnios, enterocolitis and hemorrhagiae). The objective of nanotechnology, in this case, would be to use molecules of a larger size that do not cross the placenta and act focally on the uterus [35][52]. A good example is the work of Paul et al. [36][53], by developing immunoliposomes (liposomes that use antibodies for targeting a specific organ or condition) loaded with tocolytic drugs and conjugated to oxytocin receptor antibody, for targeting the oxytocin receptor on the pregnant uterus.
However, fetal treatment with nanoparticles is at its very early beginning and, having in mind possible harmful effects, extensive preclinical studies should be undertaken prior to its practical application.
