Approximately 71 million people worldwide are infected with the hepatitis C virus (HCV). Injectable drug use represents the most common route of transmission in Europe and other developed countries. We studied the molecular characteristics of the HCV infection among mono-infected people who used drugs (PWUD) in Italy. Among 208 PWUD with anti-HCV antibodies, 101 (48.6%) were HCV RNA-positive, the majority (47%) were infected with the HCV genotype (Gt)1a, followed by Gt3a (34.9%), Gt4 (9.1%), Gt1b (4.5%), and Gt2 (4.5%). Bayesian phylogenetic analyses of clustered HCV NS5B sequences from 66 HCV-positive PWUDs with available plasma samples indicated age and neighborhood proximity as the most common characteristics between closely related HCV strains. Population dynamics, as measured by a coalescent Bayesian skyline analysis, revealed an increase in HCV Gt1a infections from the mid-1980s to mid-1990s. While HCV Gt3a infections were first detected in the 1980s, patient numbers with this genotype subtype remained relatively constant. For both Gt1a and Gt3a, Birth–Death Bayesian Skyline analyses produced higher reproduction numbers post 2014. For earlier time intervals, slow growths were observed for both Gt1a and Gt3a with reproduction numbers (Re) of approximately 1. The evolutionary rates for Gt1a and Gt3a were estimated as 2.23 × 10−4 and 3.85 × 10−4, respectively.
- hepatitis C virus (HCV)
- HCV genotypes
- molecular epidemiology
- phylogeny
- people who use drugs (PWUD)
- viral evolution
1. Introduction
The WHO estimated that approximately 71 million people worldwide are infected with the hepatitis C virus (HCV), with 1.75 million new infections being reported every year [1]. This virus is a blood-borne pathogen. Before the screening for anti-HCV antibodies was introduced in 1990, blood transfusion was the main risk factor for HCV infection [2]. In 1993, Western countries started using a nucleic acid amplification (NAT) test to detect the presence of HCV RNA. The availability of more sensitive tests led to a reduction in HCV prevalence. In Italy in 2013, the HCV RNA incidence rate in first-time blood donors was 2.5 per 100,000 [3]. Illicit drug users (IDUs) have been the predominant HCV-infected population since the early 2000s [4]. The WHO estimated that nearly 13 million individuals worldwide are IUDs, of which 67% are living with HCV [5]. Despite the route of transmission being similar for HCV and HIV, the transmission rates appear to vary. The HCV is estimated to be approximately ten times more transmissible than HIV, as only 30% of infected IDUs have HIV. Seroprevalence of HCV antibodies is very high among people who inject drugs (PWID); up to 90% of PWID have HCV antibodies compared with approximately 10% with HIV seroprevalence [6][7]. Preventive strategies, such as the reduction of syringe exchange with better aseptic technique compliance, have facilitated the reduction of HCV transmission by drug injection. However, numerous studies have shown that the sharing of drug paraphernalia is responsible for the spread of HCV among both IDUs and non-IDUs [8][9]. Specifically, the sharing of a sniffer device is a relevant factor in the spread of HCV among non-IDUs [10][11]. Changes in drug consumption and the awareness of safer drug injection practices have reduced the incidence of HCV transmission. In France, for instance, the cumulative incidence of HCV infection in IDUs was 40–50% over the last five years with the implementation of safer drug practices; this is compared with 90% in the early 1990s [12][13]. In Italy, in 2016, a national surveillance analysis at the Italian Centers for substance dependence indicated that approximately half a million of the population were people who use drugs (PWUD) and 30% tested positive for HCV [14]. The HCV status of these patients remains poorly understood, especially the variability in HCV transmission dynamics among this special population. As reported for other Western countries [15], genotypes 1a (Gt1a) and 3 (Gt3) are the most prevalent among PWID. HCV Gt4 prevalence is also increasing and drug use seems to be a key factor for the spread of this genotype [16], combined with people emigrating from African and Asian countries. To date, no phylogenetic or phylodynamic studies have been performed that focus on the correlation of drug use with the HCV genotype and the age of the patient.2. CDiscurrent Analysis on HCV Infectssion
In this study, HCV infection among PWUD was evaluated around the metropolitan area of Rome. HCV RNA was detected in 48.6% of participants who tested positive for anti-HCV antibodies. This prevalence was considerably higher when compared with reports for the general population, although slightly less than reported in 2009 by Stroffolini et al. where 68.3% of HCV-positive IDUs were also viremic [16]. We could not associate HCV RNA prevalence with specific age groups since 82% of the study participants were >45 years old. Gt1b was infrequently observed (prevalence, 4.5%) in comparison to the general population [17], suggesting a different route of transmission. The prevalence of Gt4 (9.1%) was in line with previously described results over the past few decades in Italy and other Mediterranean basin countries [18][19][20]. In fact, the prevalence of this genotype in Europe ranges from 8.2% to 20% [21][22]. In France, the prevalence of Gt4 has increased from 4% in 1990 to greater than 11% over the last decade [23][24]. In Greece, Gt4 accounts for approximately 15% of all HCV infections [25][26]. Furthermore, our results showed a reduced prevalence of Gt4 when compared with other certain populations, such as men who have sex with men [27]. Gt1a was the most prevalent genotype (47%) in all evaluated age categories, followed by Gt3a (34.9%). In line with our results, Stroffolini et al. reported that the most common HCV genotypes among intravenous IDUs with Gt1a and Gt3a prevalence of 47.9% and 39.7%, respectively [16]. The marked differences between Gt1a and Gt1b strengthen the hypothesis that these two subtypes have different transmission routes. Gt1a was first introduced into Italy in the early 1970s, and coalescent Bayesian skyline analysis highlighted an exponential increase in Gt1a incidence between the 1980s and 1990s (Figure 1). This finding is in accordance with the younger age of infected HCV Gt1a patients, especially when compared with Gt1b, the main genotype detected in older chronically infected HCV patients [15]. Thus, there appears to be a dispersal origin. In the majority of cases, PWUD strains are intermixed with non-PWUD strains suggesting that Gt1a became a widely distributed subtype, affecting different patient demographics. The Gt1a sequences formed distinct clusters, distributed throughout the Bayesian phylogenetic tree without any distinct pattern. This is likely the result of a widespread and long-term distribution of Gt1a within the global population, and it is consistent with observations such as those in Greece regarding Gt1a dispersal among IDUs [28].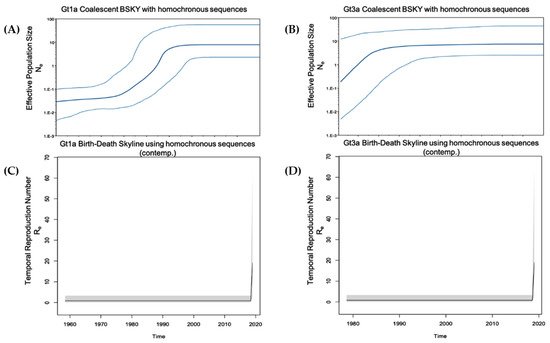
| Characteristics | Total (n) | HCV Genotype | ||||
|---|---|---|---|---|---|---|
| 1a | 1b | 2 | 3a | 4 | ||
| Age (years), mean ± SD | 50 ± 8.9 (n = 66) | 49 ± 9.4 (n = 31) | 57 ± 3.5 (n = 3) | 61 ± 5.3 (n = 3) | 48 ± 9.1 (n = 23) | 52 ± 2.3 (n = 6) |
| ≤34 | 5 | 3 | - | - | 2 | - |
| 35–44 | 7 | 4 | - | - | 3 | - |
| ≥45 | 54 | 24 | 3 | 3 | 18 | 6 |
| Gender | (n = 66) | (n = 31) | (n = 3) | (n = 3) | (n = 23) | (n = 6) |
| Male | 54 | 26 | 3 | 3 | 18 | 4 |
| Female | 12 | 5 | - | - | 5 | 2 |
| Estimated year of HCV infection | (n = 66) | (n = 31) | (n = 3) | (n = 3) | (n = 23) | (n = 6) |
| <1990 | 7 | 5 | - | - | 1 | 1 |
| 1990–1995 | 22 | 8 | 1 | 1 | 9 | 3 |
| 1996–2001 | 13 | 6 | 1 | 1 | 4 | 1 |
| 2002–2007 | 7 | 1 | 1 | - | 4 | 1 |
| 2008–2013 | 10 | 7 | - | - | 3 | - |
| 2014–2019 | 6 | 3 | - | 1 | 2 | - |
| NS | 1 | 1 | - | - | - | - |
| Assumption for infection | (n = 66) | (n = 31) | (n = 3) | (n = 3) | (n = 23) | (n = 6) |
| Inhalation | 4 | 2 | - | - | 1 | 1 |
| Intravenous | 5 | 4 | - | - | 1 | - |
| Inhal and Intrav | 55 | 23 | 3 | 3 | 21 | 5 |
| NS | 2 | 2 | - | - | - | |
| Most commonly injected drug at recruitment | (n = 66) | (n = 31) | (n = 3) | (n = 3) | (n = 23) | (n = 6) |
| Heroin | 20 | 9 | - | 2 | 8 | 1 |
| Cocaine | 1 | 1 | - | - | - | - |
| Heroin and Cocaine | 45 | 21 | 3 | 1 | 15 | 5 |
References
- World Health Organization (WHO). Global Hepatitis Report. 2017. Available online: (accessed on 15 March 2021).
- Ramadori, G.; Meier, V. Hepatitis C virus infection: 10 years after the discovery of the virus. Eur. J. Gastroenterol. Hepatol. 2001, 13, 465–471.
- Piccinini, V.; Facco, G.; Catalano, L.; Pupella, S.; Grazzini, G. Transfusion Transmitted Infections in Italy: Blood Donors Epidemiological Surveillance. Report 2013. Available online: (accessed on 15 March 2021).
- Sherman, K.E.; Rouster, S.D.; Chung, R.T.; Rajicic, N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: A cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin. Infect. Dis. 2002, 34, 831–837.
- World Health Organization (WHO). 17 Million People with Access to Antiretroviral Therapy. 2016. Available online: (accessed on 15 March 2021).
- Walsh, N.; Maher, L. HIV and viral hepatitis C coinfection in people who inject drugs. Curr. Opin. HIV AIDS 2012, 7, 339–344.
- Doyle, M.; Maher, L.; Graham, S.; Wand, H.; Iversen, J. Hepatitis C virus prevalence and associated risk factors among Indigenous Australians who inject drugs. Aust. N. Z. J. Public Health 2018, 42, 52–56.
- Aaron, S.; McMahon, J.M.; Milano, D.; Torres, L.; Clatts, M.; Tortu, S.; Mildvan, D.; Simm, M. Intranasal transmission of hepatitis C virus: Virological and clinical evidence. Clin. Infect. Dis. 2008, 47, 931–934.
- Oliveira-Filho, A.B.; Sawada, L.; Pinto, L.C.; Locks, D.; Bahia, S.L.; Castro, J.A.; Hermes, R.B.; Brasil-Costa, I.; Amaral, C.E.; Lemos, J.A. Epidemiological aspects of HCV infection in non-injecting drug users in the Brazilian state of Pará, eastern Amazon. Virol. J. 2014, 11, 38.
- Caiaffa, W.T.; Zocratto, K.F.; Osimani, M.L.; Martinez, P.L.; Radulich, G.; Latorre, L.; Muzzio, E.; Segura, M.; Chiparelli, H.; Russi, J.; et al. Hepatitis C virus among non-injecting cocaine users (NICUs) in South America: Can injectors be a bridge? Addiction 2011, 106, 143–151.
- Oliveira-Filho, A.B.; Sawada, L.; Pinto, L.C.; Locks, D.; Bahia, S.L.; Brasil-Costa, I.; Lemos, J.A. HCV infection among cocaine users in the state of Para, Brazilian Amazon. Arch. Virol. 2013, 158, 1555–1560.
- Chossegros, P. Prise en charge de la toxicomanie en France (une histoire) [Management of drug addiction in France (a short history)]. Gastroenterol. Clin. Biol. 2007, 31 Pt 3, 4S44–4S50. (In French)
- Lucidarme, D.; Foutrein, P.; Creusy, C.; Forzy, G.; Foutrein-Comes, M.C.; Muyssen, A.; Bailly, D.; Parquet, P.J.; Filoche, B. Prévalence des marqueurs des hépatites C, B et D et aspects histopathologiques dans un groupe de toxicomanes intraveineux [Prevalence of hepatitis C, B and D markers and histopathological aspects in a group of intravenous drug addicts]. Gastroenterol. Clin. Biol. 1994, 18, 964–968.
- Dipartimento per le Politiche Antidroga. Relazione Annuale al Parlamento sul Fenomeno Delle Tossicodipendenze in Italia Anno 2017 (dati 2016). 2017. Available online: (accessed on 15 March 2021). (In Italian)
- Robaeys, G.; Bielen, R.; Azar, D.G.; Razavi, H.; Nevens, F. Global genotype distribution of hepatitis C viral infection among people who inject drugs. J. Hepatol. 2016, 65, 1094–1103.
- Stroffolini, T.; D’Egidio, P.F.; Aceti, A.; Filippini, P.; Puoti, M.; Leonardi, C.; Almasio, P.L. DAVIS drug addicted, HCV prevalence in Italy an epidemiological, observational, cross-sectional, multicenter study participating centers. Hepatitis C virus infection among drug addicts in Italy. J. Med. Virol. 2012, 84, 1608–1612.
- Alberti, A.; Lacoin, L.; Morais, E.; Lefevre, C.; Abogunrin, S.; Iheanacho, I. Literature review of the distribution of hepatitis C virus genotypes across Europe. J. Med. Virol. 2016, 88, 2157–2169.
- Ciccozzi, M.; Lo Presti, A.; Ciccaglione, A.R.; Zehender, G.; Ciotti, M. Phylogeny and phylodinamic of Hepatitis C in Italy. BMC Infect. Dis. 2012, 12 (Suppl. 2), S5.
- Guadagnino, V.; Stroffolini, T.; Caroleo, B.; Menniti Ippolito, F.; Rapicetta, M.; Ciccaglione, A.R.; Chionne, P.; Madonna, E.; Costantino, A.; De Sarro, G.; et al. Hepatitis C virus infection in an endemic area of Southern Italy 14 years later: Evidence for a vanishing infection. Dig. Liver Dis. 2013, 45, 403–407.
- Matera, G.; Lamberti, A.; Quirino, A.; Focà, D.; Giancotti, A.; Barreca, G.S.; Guadagnino, V.; Liberto, M.C. Changes in the prevalence of hepatitis C virus (HCV) genotype 4 in Calabria, Southern Italy. Diagn. Microbiol. Infect. Dis. 2002, 42, 169–173.
- Fernández-Arcás, N.; López-Siles, J.; Trapero, S.; Ferraro, A.; Ibáñez, A.; Orihuela, F.; Maldonado, J.; Alonso, A. High prevalence of hepatitis C virus subtypes 4c and 4d in Malaga (Spain): Phylogenetic and epidemiological analyses. J. Med. Virol. 2006, 78, 1429–1435.
- Poveda, E.; Wyles, D.L.; Mena, A.; Pedreira, J.D.; Castro-Iglesias, A.; Cachay, E. Update on hepatitis C virus resistance to direct-acting antiviral agents. Antivir. Res. 2014, 108, 181–191.
- Kamal, S.M.; Nasser, I.A. Hepatitis C genotype 4: What we know and what we don’t yet know. Hepatology 2008, 47, 1371–1383.
- Payan, C.; Roudot-Thoraval, F.; Marcellin, P.; Bled, N.; Duverlie, G.; Fouchard-Hubert, I.; Trimoulet, P.; Couzigou, P.; Cointe, D.; Chaput, C.; et al. Changing of hepatitis C virus genotype patterns in France at the beginning of the third millenium: The GEMHEP GenoCII Study. J. Viral Hepat. 2005, 12, 405–413.
- Katsoulidou, A.; Sypsa, V.; Tassopoulos, N.C.; Boletis, J.; Karafoulidou, A.; Ketikoglou, I.; Tsantoulas, D.; Vafiadi, I.; Hatzis, G.; Skoutelis, A.; et al. Molecular epidemiology of hepatitis C virus (HCV) in Greece: Temporal trends in HCV genotype-specific incidence and molecular characterization of genotype 4 isolates. J. Viral Hepat. 2006, 13, 19–27.
- Savvas, S.P.; Koskinas, J.; Sinani, C.; Hadziyannis, A.; Spanou, F.; Hadziyannis, S.J. Changes in epidemiological patterns of HCV infection and their impact on liver disease over the last 20 years in Greece. J. Viral Hepat. 2005, 12, 551–557.
- van de Laar, T.; Pybus, O.; Bruisten, S.; Brown, D.; Nelson, M.; Bhagani, S.; Vogel, M.; Baumgarten, A.; Chaix, M.L.; Fisher, M.; et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology 2009, 136, 1609–1617.
- Papachristou, E.; Tsagkovits, A.; Zavitsanou, A.; Hatzakis, A.; Paraskevis, D. HCV dispersal patterns among intravenous drug users (IDUs) in Athens metropolitan area. Infect. Genet. Evol. 2016, 45, 415–419.
- Lucidarme, D.; Duburque, C.; Bulois, P.; Filoche, B. Evolution of HCV incidence in drug users in France. Epidemiol. Infect. 2011, 139, 1287–1295.
- Tarallo, P. Incontri Indiani 2015. Available online: (accessed on 12 March 2021).
- World Drug Report. The Global Heroin Market. 2010. Available online: (accessed on 12 March 2021).
- Manuylov, V.A.; Chub, E.V.; Kichatova, V.S.; Soboleva, N.V.; Isaeva, O.V.; Zamyatnin, A.A., Jr.; Netesov, S.V. Hepatitis C virus subtype 3a was introduced in the USSR in the early 1980s. J. Gen. Virol. 2017, 98, 2079–2087.
- Lehmann, M.; Meyer, M.F.; Monazahian, M.; Tillmann, H.L.; Manns, M.P.; Wedemeyer, H. High rate of spontaneous clearance of acute hepatitis C virus genotype 3 infection. J. Med. Virol. 2004, 73, 387–391.
- Chan, A.; Patel, K.; Naggie, S. Genotype 3 Infection: The Last Stand of Hepatitis C Virus. Drugs 2017, 77, 131–144.
- Mwangi, J.; Nganga, Z.; Mpoke, S.; Lihana, R.; Kinyua, J.; Lagat, N.; Muriuki, J.; Lel, R.; Kageha, S.; Osman, S.; et al. Hepatitis C virus genotypes in Kenya. Arch. Virol. 2015, 161, 95–101.
- Ramia, S.; Eid-Fares, J. Distribution of hepatitis C virus genotypes in the Middle East. Int. J. Infect. Dis. 2006, 10, 272–277.
