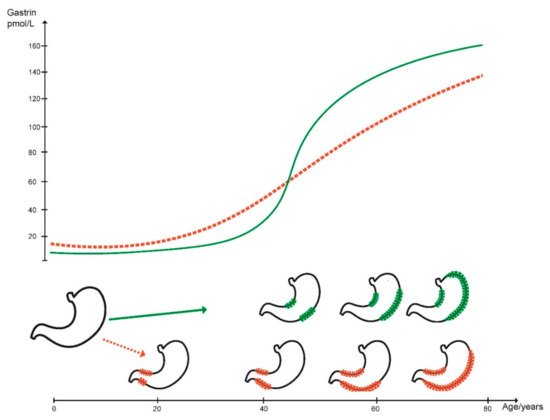
Gastric cancer is still an important disease causing many deaths worldwide, although there has been a marked reduction in prevalence during the last few decades. The decline in gastric cancer prevalence is due to a reduction in Helicobacter pylori infection which has occurred for at least 50 years. The most probable mechanism for the carcinogenic effect of H. pylori is hypergastrinemia since H. pylori infected individuals do not have increased risk of gastric cancer before the development of oxyntic atrophy. When atrophy has developed, the carcinogenic process continues independent of H. pylori. Autoimmune gastritis also induces oxyntic atrophy leading to marked hypergastrinemia and development of ECL cell neoplasia as well as adenocarcinoma. Similarly, long-term treatment with efficient inhibitors of acid secretion like the proton pump inhibitors (PPIs) predisposes to ECL cell neoplasia of a different degree of malignancy. Contrasting the colon where most cancers develop from polyps, most polyps in the stomach have a low malignant potential. Nevertheless, gastric polyps may also give rise to cancer and have some risk factors and mechanisms in common with gastric cancer.
- ECL cell
- gastrin
- gastric cancer
- gastric polyps
- gastritis
1. Introduction
2. Gastric Cancer
3. Gastric Polyps
3.1. Hyperplastic Polyps
Hyperplastic polyps (HPs) are prevalent and develop in association with gastritis and gastric atrophy [80][81] or may develop in response to injury that stimulates regeneration and proliferation. The histological appearance of hyperplastic polyps overlaps with polyps that have previously been characterised as inflammatory, the latter term now considered a misnomer [82]. HPs are most often single and may be found in all parts of the stomach. Previously, HPs were most often found as single polyps in the antrum, whereas there has been described a shift towards a more proximal location for HPs as well as for other gastric polyps [83]. Sporadic hyperplastic polyps cannot be distinguished morphologically from polyps occurring in syndromes with hyperplastic polyposis [78]. The sporadic HPs are associated with gastritis and atrophy, the same conditions being central in gastric carcinogenesis discussed above. Hyperplastic polyps have repeatedly been found to regress after H. pylori eradication, demonstrating the causal role of H. pylori [84]. There is an increased risk of gastric neoplasia in patients with HPs [85]. The mechanism by which HPs develop through presumed proliferation of the foveolar surface mucosa is not known, but since they are localised, there must be a local change in the growth regulation or a mutation in the cell of origin affecting the proliferation rate. HPs also develop in the cardia [86] of patients with gastroesophageal reflux disease (GERD). Hypertrophic gastropathy in Ménétriers disease, a condition of uncertain etiology, but with increased risk of gastric cancer development, may also contain elements that mimic HPs [87].3.2. Adenomatous Polyps
3.3. Fundic Gland Polyps
3.4. Gastric Polyps in Patients with Portal Hypertension
4. Conclusions
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Parsonnet, J.; Friedman, G.D.; Vandersteen, D.P.; Chang, Y.; Vogelman, J.H.; Orentreich, N.; Sibley, R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991, 325, 1127–1131.
- Waldum, H.L.; Fossmark, R. Types of Gastric Carcinomas. Int. J. Mol. Sci. 2018, 19, 4109.
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attemt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49.
- Tang, C.T.; Zeng, L.; Yang, J.; Zeng, C.; Chen, Y. Analysis of the Incidence and Survival of Gastric Cancer Based on the Lauren Classification: A Large Population-Based Study Using SEER. Front. Oncol. 2020, 10, 1212.
- Schauer, M.; Peiper, M.; Theisen, J.; Knoefel, W. Prognostic factors in patients with diffuse type gastric cancer (linitis plastica) after operative treatment. Eur. J. Med. Res. 2011, 16, 29–33.
- Sheweita, S.A.; Alsamghan, A.S. Molecular Mechanisms Contributing Bacterial Infections to the Incidence of Various Types of Cancer. Mediators Inflamm. 2020, 2020, 4070419.
- Park, J.Y.; Forman, D.; Waskito, L.A.; Yamaoka, Y.; Crabtree, J.E. Epidemiology of Helicobacter pylori and CagA-Positive Infections and Global Variations in Gastric Cancer. Toxins 2018, 10, 163.
- Peek, R.M., Jr.; Miller, G.G.; Tham, K.T.; Perez-Perez, G.I.; Zhao, X.; Atherton, J.C.; Blaser, M.J. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab. Investig. 1995, 73, 760–770.
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Virulence Factor Cytotoxin-Associated Gene A (CagA)-Mediated Gastric Pathogenicity. Int. J. Mol. Sci. 2020, 21, 7430.
- Hatakeyama, M. Helicobacter pylori CagA and gastric cancer: A paradigm for hit-and-run carcinogenesis. Cell Host Microbe 2014, 15, 306–316.
- Bauer, M.; Nascakova, Z.; Mihai, A.I.; Cheng, P.F.; Levesque, M.P.; Lampart, S.; Hurwitz, R.; Pfannkuch, L.; Dobrovolna, J.; Jacobs, M.; et al. The ALPK1/TIFA/NF-κB axis links a bacterial carcinogen to R-loop-induced replication stress. Nat. Commun. 2020, 11, 5117.
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789.
- Negrini, R.; Savio, A.; Poiesi, C.; Appelmelk, B.J.; Buffoli, F.; Paterlini, A.; Cesari, P.; Graffeo, M.; Vaira, D.; Franzin, G. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology 1996, 111, 655–665.
- Faller, G.; Steininger, H.; Appelmelk, B.; Kirchner, T. Evidence of novel pathogenic pathways for the formation of antigastric autoantibodies in Helicobacter pylori gastritis. J. Clin. Pathol. 1998, 51, 244–245.
- Rowland, M.; Daly, L.; Vaughan, M.; Higgins, A.; Bourke, B.; Drumm, B. Age-specific incidence of Helicobacter pylori. Gastroenterology 2006, 130, 65–72.
- Haruma, K.; Kamada, T.; Kawaguchi, H.; Okamoto, S.; Yoshihara, M.; Sumii, K.; Inoue, M.; Kishimoto, S.; Kajiyama, G.; Miyoshi, A. Effect of age and Helicobacter pylori infection on gastric acid secretion. J. Gastroenterol. Hepatol. 2000, 15, 277–283.
- Waldum, H.; Mjønes, P. Towards Understanding of Gastric Cancer Based upon Physiological Role of Gastrin and ECL Cells. Cancers 2020, 12, 3477.
- Strickland, R.G.; Bhathal, P.S.; Korman, M.G.; Hansky, J. Serum gastrin and the antral mucosa in atrophic gastritis. Br. Med. J. 1971, 4, 451–453.
- Borch, K.; Renvall, H.; Liedberg, G. Gastric endocrine cell hyperplasia and carcinoid tumors in pernicious anemia. Gastroenterology 1985, 88, 638–648.
- Zamcheck, N.; Grable, E.; Ley, A.; Norman, L. Occurrence of gastric cancer among patients with pernicious anemia at the Boston City Hospital. N. Engl. J. Med. 1955, 252, 1103–1110.
- Bizzaro, N.; Antico, A.; Villalta, D. Autoimmunity and Gastric Cancer. Int. J. Mol. Sci. 2018, 19, 377.
- Sato, Y.; Iwafuchi, M.; Ueki, J.; Yoshimura, A.; Mochizuki, T.; Motoyama, H.; Sugimura, K.; Honma, T.; Narisawa, R.; Ichida, T.; et al. Gastric carcinoid tumors without autoimmune gastritis in Japan: A relationship with Helicobacter pylori infection. Dig. Dis. Sci. 2002, 47, 579–585.
- Antonodimitrakis, P.; Tsolakis, A.; Welin, S.; Kozlovacki, G.; Oberg, K.; Granberg, D. Gastric carcinoid in a patient infected with Helicobacter pylori: A new entity? World J. Gastroenterol. 2011, 17, 3066–3068.
- Hansson, L.R.; Engstrand, L.; Nyren, O.; Lindgren, A. Prevalence of Helicobacter pylori infection in subtypes of gastric cancer. Gastroenterology 1995, 109, 885–888.
- Kim, J.Y.; Lee, H.S.; Kim, N.; Shin, C.M.; Lee, S.H.; Park, Y.S.; Hwang, J.H.; Kim, J.W.; Jeong, S.H.; Lee, D.H.; et al. Prevalence and clinicopathologic characteristics of gastric cardia cancer in South Korea. Helicobacter 2012, 17, 358–368.
- Sjöblom, S.M.; Sipponen, P.; Miettinen, M.; Karonen, S.L.; Jrvinen, H.J. Gastroscopic screening for gastric carcinoids and carcinoma in pernicious anemia. Endoscopy 1988, 20, 52–56.
- Qvigstad, G.; Qvigstad, T.; Westre, B.; Sandvik, A.K.; Brenna, E.; Waldum, H.L. Neuroendocrine differentiation in gastric adenocarcinomas associated with severe hypergastrinemia and/or pernicious anemia. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2002, 110, 132–139.
- Bakke, I.; Qvigstad, G.; Brenna, E.; Sandvik, A.K.; Waldum, H.L. Gastrin has a specific proliferative effect on the rat enterochromaffin-like cell, but not on the parietal cell: A study by elutriation centrifugation. Acta Physiol. Scand. 2000, 169, 29–37.
- Tielemans, Y.; Chen, D.; Sundler, F.; Håkanson, R.; Willems, G. Reversibility of the cell kinetic changes induced by omeprazole in the rat oxyntic mucosa. An autoradiographic study using tritiated thymidine. Scand. J. Gastroenterol. 1992, 27, 155–160.
- Fukui, H.; Kinoshita, Y.; Maekawa, T.; Okada, A.; Waki, S.; Hassan, S.; Okamoto, H.; Chiba, T. Regenerating gene protein may mediate gastric mucosal proliferation induced by hypergastrinemia in rats. Gastroenterology 1998, 115, 1483–1493.
- Miyaoka, Y.; Kadowaki, Y.; Ishihara, S.; Ose, T.; Fukuhara, H.; Kazumori, H.; Takasawa, S.; Okamoto, H.; Chiba, T.; Kinoshita, Y. Transgenic overexpression of Reg protein caused gastric cell proliferation and differentiation along parietal cell and chief cell lineages. Oncogene 2004, 23, 3572–3579.
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992, 52, 6735–6740.
- Spence, A.D.; Cardwell, C.R.; McMenamin, Ú.C.; Hicks, B.M.; Johnston, B.T.; Murray, L.J.; Coleman, H.G. Adenocarcinoma risk in gastric atrophy and intestinal metaplasia: A systematic review. BMC Gastroenterol. 2017, 17, 157.
- Kinoshita, H.; Hayakawa, Y.; Koike, K. Metaplasia in the Stomach-Precursor of Gastric Cancer? Int. J. Mol. Sci. 2017, 18, 2063.
- Graham, D.Y.; Zou, W.Y. Guilt by association: Intestinal metaplasia does not progress to gastric cancer. Curr. Opin. Gastroenterol. 2018, 34, 458–464.
- Blair, A.J., 3rd; Richardson, C.T.; Walsh, J.H.; Feldman, M. Variable contribution of gastrin to gastric acid secretion after a meal in humans. Gastroenterology 1987, 92, 944–949.
- Jianu, C.S.; Fossmark, R.; Viset, T.; Qvigstad, G.; Sordal, O.; Marvik, R.; Waldum, H.L. Gastric carcinoids after long-term use of a proton pump inhibitor. Aliment. Pharmacol. Ther. 2012, 36, 644–649.
- Cavalcoli, F.; Zilli, A.; Conte, D.; Ciafardini, C.; Massironi, S. Gastric neuroendocrine neoplasms and proton pump inhibitors: Fact or coincidence? Scand. J. Gastroenterol. 2015, 50, 1397–1403.
- Nandy, N.; Hanson, J.A.; Strickland, R.G.; McCarthy, D.M. Solitary Gastric Carcinoid Tumor Associated with Long-Term Use of Omeprazole: A Case Report and Review of the Literature. Dig. Dis. Sci. 2016, 61, 708–712.
- Lamberts, R.; Creutzfeldt, W.; Stockmann, F.; Jacubaschke, U.; Maas, S.; Brunner, G. Long-term omeprazole treatment in man: Effects on gastric endocrine cell populations. Digestion 1988, 39, 126–135.
- Jianu, C.S.; Lange, O.J.; Viset, T.; Qvigstad, G.; Martinsen, T.C.; Fougner, R.; Kleveland, P.M.; Fossmark, R.; Hauso, Ø.; Waldum, H.L. Gastric neuroendocrine carcinoma after long-term use of proton pump inhibitor. Scand. J. Gastroenterol. 2012, 47, 64–67.
- Calvete, O.; Reyes, J.; Zuniga, S.; Paumard-Hernandez, B.; Fernandez, V.; Bujanda, L.; Rodriguez-Pinilla, M.S.; Palacios, J.; Heine-Suner, D.; Banka, S.; et al. Exome sequencing identifies ATP4A gene as responsible of an atypical familial type I gastric neuroendocrine tumour. Hum. Mol. Genet. 2015, 24, 2914–2922.
- Fossmark, R.; Calvete, O.; Mjones, P.; Benitez, J.; Waldum, H.L. ECL-cell carcinoids and carcinoma in patients homozygous for an inactivating mutation in the gastric H(+) K(+) ATPase alpha subunit. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2016, 124, 561–566.
- Solcia, E.; Rindi, G.; Silini, E.; Villani, L. Enterochromaffin-like (ECL) cells and their growths: Relationships to gastrin, reduced acid secretion and gastritis. Baillieres Clin. Gastroenterol. 1993, 7, 149–165.
- Solcia, E.; Capella, C.; Buffa, R.; Usellini, L.; Frigerio, B.; Fontana, P. Endocrine cells of the gastrointestinal tract and related tumors. Pathobiol. Annu. 1979, 9, 163–204.
- Moayyedi, P.; Eikelboom, J.W.; Bosch, J.; Connolly, S.J.; Dyal, L.; Shestakovska, O.; Leong, D.; Anand, S.S.; Stork, S.; Branch, K.R.H.; et al. Safety of Proton Pump Inhibitors Based on a Large, Multi-Year, Randomized Trial of Patients Receiving Rivaroxaban or Aspirin. Gastroenterology 2019, 157, 682–691.
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Wong, I.C.K.; Leung, W.K. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: A population-based study. Gut 2018, 67, 28–35.
- Cheung, K.S.; Leung, W.K. Long-term use of proton-pump inhibitors and risk of gastric cancer: A review of the current evidence. Ther. Adv. Gastroenterol. 2019, 12, 1756284819834511.
- Brusselaers, N.; Wahlin, K.; Engstrand, L.; Lagergren, J. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: A nationwide population-based cohort study in Sweden. BMJ Open 2017, 7, e017739.
- Bergquist, J.R.; Leiting, J.L.; Habermann, E.B.; Cleary, S.P.; Kendrick, M.L.; Smoot, R.L.; Nagorney, D.M.; Truty, M.J.; Grotz, T.E. Early-onset gastric cancer is a distinct disease with worrisome trends and oncogenic features. Surgery 2019, 166, 547–555.
- Waldum, H.L. The increase in early-onset gastric carcinomas from 1995 is probably due to the introduction of proton pump inhibitors. Surgery 2020, 168, 568–569.
- Kuipers, E.J.; Lundell, L.; Klinkenberg-Knol, E.C.; Havu, N.; Festen, H.P.; Liedman, B.; Lamers, C.B.; Jansen, J.B.; Dalenback, J.; Snel, P.; et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N. Engl. J. Med. 1996, 334, 1018–1022.
- Meuwissen, S.G.; Craanen, M.E.; Kuipers, E.J. Gastric mucosal morphological consequences of acid suppression: A balanced view. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 497–510.
- Schenk, B.E.; Kuipers, E.J.; Klinkenberg-Knol, E.C.; Bloemena, E.; Nelis, G.F.; Festen, H.P.; Jansen, E.H.; Biemond, I.; Lamers, C.B.; Meuwissen, S.G. Hypergastrinaemia during long-term omeprazole therapy: Influences of vagal nerve function, gastric emptying and Helicobacter pylori infection. Aliment. Pharmacol. Ther. 1998, 12, 605–612.
- Havu, N. Enterochromaffin-like cell carcinoids of gastric mucosa in rats after life-long inhibition of gastric secretion. Digestion 1986, 35 (Suppl. 1), 42–55.
- Poynter, D.; Selway, S.A.; Papworth, S.A.; Riches, S.R. Changes in the gastric mucosa of the mouse associated with long lasting unsurmountable histamine H2 blockade. Gut 1986, 27, 1338–1346.
- Martinsen, T.C.; Kawase, S.; Hakanson, R.; Torp, S.H.; Fossmark, R.; Qvigstad, G.; Sandvik, A.K.; Waldum, H.L. Spontaneous ECL cell carcinomas in cotton rats: Natural course and prevention by a gastrin receptor antagonist. Carcinogenesis 2003, 24, 1887–1896.
- Cadiot, G.; Vissuzaine, C.; Potet, F.; Mignon, M. Fundic argyrophil carcinoid tumor in a patient with sporadic-type Zollinger-Ellison syndrome. Dig. Dis. Sci. 1995, 40, 1275–1278.
- Solcia, E.; Capella, C.; Fiocca, R.; Rindi, G.; Rosai, J. Gastric argyrophil carcinoidosis in patients with Zollinger-Ellison syndrome due to type 1 multiple endocrine neoplasia. A newly recognized association. Am. J. Surg. Pathol. 1990, 14, 503–513.
- Bordi, C.; Senatore, S.; Missale, G. Gastric carcinoid following gastrojejunostomy. Am. J. Dig. Dis. 1976, 21, 667–671.
- Boyce, M.; Moore, A.R.; Sagatun, L.; Parsons, B.N.; Varro, A.; Campbell, F.; Fossmark, R.; Waldum, H.L.; Pritchard, D.M. Netazepide, a gastrin/cholecystokinin-2 receptor antagonist, can eradicate gastric neuroendocrine tumours in patients with autoimmune chronic atrophic gastritis. Br. J. Clin. Pharmacol. 2017, 83, 466–475.
- Fossmark, R.; Sordal, O.; Jianu, C.S.; Qvigstad, G.; Nordrum, I.S.; Boyce, M.; Waldum, H.L. Treatment of gastric carcinoids type 1 with the gastrin receptor antagonist netazepide (YF476) results in regression of tumours and normalisation of serum chromogranin A. Aliment. Pharmacol. Ther. 2012, 36, 1067–1075.
- Poulsen, A.H.; Christensen, S.; McLaughlin, J.K.; Thomsen, R.W.; Sørensen, H.T.; Olsen, J.H.; Friis, S. Proton pump inhibitors and risk of gastric cancer: A population-based cohort study. Br. J. Cancer 2009, 100, 1503–1507.
- Seo, S.I.; Park, C.H.; You, S.C.; Kim, J.Y.; Lee, K.J.; Kim, J.; Kim, Y.; Yoo, J.J.; Seo, W.W.; Lee, H.S.; et al. Association between proton pump inhibitor use and gastric cancer: A population-based cohort study using two different types of nationwide databases in Korea. Gut 2021.
- Waldum, H.L.; Sørdal, Ø.; Fossmark, R. Proton pump inhibitors (PPIs) may cause gastric cancer—Clinical consequences. J. Gastroenterol. 2018, 53, 639–642.
- Qvigstad, G.; Arnestad, J.S.; Brenna, E.; Waldum, H.L. Treatment with proton pump inhibitors induces tolerance to histamine-2 receptor antagonists in Helicobacter pylori-negative patients. Scand. J. Gastroenterol. 1998, 33, 1244–1248.
- Wölffling, S.; Daddi, A.A.; Imai-Matsushima, A.; Fritsche, K.; Goosmann, C.; Traulsen, J.; Lisle, R.; Schmid, M.; Del Mar Reines-Benassar, M.; Pfannkuch, L.; et al. EGF and BMPs govern differentiation and patterning in human gastric glands. Gastroenterology 2021.
- Kishikawa, H.; Ojiro, K.; Nakamura, K.; Katayama, T.; Arahata, K.; Takarabe, S.; Miura, S.; Kanai, T.; Nishida, J. Previous Helicobacter pylori infection-induced atrophic gastritis: A distinct disease entity in an understudied population without a history of eradication. Helicobacter 2020, 25, e12669.
- Okada, K.; Suzuki, S.; Naito, S.; Yamada, Y.; Haruki, S.; Kubota, M.; Nakajima, Y.; Shimizu, T.; Ando, K.; Uchida, Y.; et al. Incidence of metachronous gastric cancer in patients whose primary gastric neoplasms were discovered after Helicobacter pylori eradication. Gastrointest. Endosc. 2019, 89, 1152–1159.
- Algin, E.; Baykara, M.; Yilmaz, G.; Cetin, B.; Ekinci, O.; Uner, A.; Ozet, A. Is there any relationship between Helicobacter pylori infection and human epidermal growth factor receptor 2 expression in gastric cancer? J. Cancer Res. Ther. 2020, 16, S128–S132.
- Leushacke, M.; Tan, S.H.; Wong, A.; Swathi, Y.; Hajamohideen, A.; Tan, L.T.; Goh, J.; Wong, E.; Denil, S.; Murakami, K.; et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat. Cell Biol. 2017, 19, 774–786.
- Take, S.; Mizuno, M.; Ishiki, K.; Kusumoto, C.; Imada, T.; Hamada, F.; Yoshida, T.; Yokota, K.; Mitsuhashi, T.; Okada, H. Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. J. Gastroenterol. 2020, 55, 281–288.
- Ness-Jensen, E.; Bringeland, E.A.; Mattsson, F.; Mjønes, P.; Lagergren, J.; Grønbech, J.E.; Waldum, H.L.; Fossmark, R. Hypergastrinemia is associated with an increased risk of gastric adenocarcinoma with proximal location: A prospective population-based nested case-control study. Int. J. Cancer 2021, 148, 1879–1886.
- Choi, E.; Roland, J.T.; Barlow, B.J.; O’Neal, R.; Rich, A.E.; Nam, K.T.; Shi, C.; Goldenring, J.R. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut 2014, 63, 1711–1720.
- Waldum, H.L.; Oberg, K.; Sordal, O.F.; Sandvik, A.K.; Gustafsson, B.I.; Mjones, P.; Fossmark, R. Not only stem cells, but also mature cells, particularly neuroendocrine cells, may develop into tumours: Time for a paradigm shift. Ther. Adv. Gastroenterol. 2018, 11, 1756284818775054.
- Alexandraki, K.I.; Spyroglou, A.; Kykalos, S.; Daskalakis, K.; Kyriakopoulos, G.; Sotiropoulos, G.C.; Kaltsas, G.A.; Grossman, A.B. Changing biological behaviour of NETs during the evolution of the disease: Progress on progression. Endocr. Relat. Cancer 2021, 28, R121–R140.
- Vos, S.; van der Post, R.S.; Brosens, L.A.A. Gastric Epithelial Polyps: When to Ponder, When to Panic. Surg. Pathol. Clin. 2020, 13, 431–452.
- Carmack, S.W.; Genta, R.M.; Schuler, C.M.; Saboorian, M.H. The current spectrum of gastric polyps: A 1-year national study of over 120,000 patients. Am. J. Gastroenterol. 2009, 104, 1524–1532.
- Abraham, S.C.; Singh, V.K.; Yardley, J.H.; Wu, T.T. Hyperplastic polyps of the stomach: Associations with histologic patterns of gastritis and gastric atrophy. Am. J. Surg. Pathol. 2001, 25, 500–507.
- Di Giulio, E.; Lahner, E.; Micheletti, A.; Milione, M.; D’Ambra, G.; Bordi, C.; Delle Fave, G.; Annibale, B. Occurrence and risk factors for benign epithelial gastric polyps in atrophic body gastritis on diagnosis and follow-up. Aliment. Pharmacol. Ther. 2005, 21, 567–574.
- Goddard, A.F.; Badreldin, R.; Pritchard, D.M.; Walker, M.M.; Warren, B. The management of gastric polyps. Gut 2010, 59, 1270–1276.
- Fan, N.N.; Yang, J.; Sun, G.; Lu, Z.S.; Ling Hu, E.Q.; Wang, X.D.; Yang, Y.S. Changes in the spectrum of gastric polyps in the Chinese population. World J. Gastroenterol. 2015, 21, 9758–9764.
- Suzuki, S.; Ohkusa, T.; Shimoi, K.; Horiuchi, T.; Fujiki, K.; Takashimizu, I. Disappearance of multiple hyperplastic polyps after the eradication of Helicobacter pylori. Gastrointest. Endosc. 1997, 46, 566–568.
- Terada, T. Malignant transformation of foveolar hyperplastic polyp of the stomach: A histopathological study. Med. Oncol. 2011, 28, 941–944.
- Melton, S.D.; Genta, R.M. Gastric cardiac polyps: A clinicopathologic study of 330 cases. Am. J. Surg. Pathol. 2010, 34, 1792–1797.
- Rich, A.; Toro, T.Z.; Tanksley, J.; Fiske, W.H.; Lind, C.D.; Ayers, G.D.; Piessevaux, H.; Washington, M.K.; Coffey, R.J. Distinguishing Ménétrier’s disease from its mimics. Gut 2010, 59, 1617–1624.
- Saito, K.; Arai, K.; Mori, M.; Kobayashi, R.; Ohki, I. Effect of Helicobacter pylori eradication on malignant transformation of gastric adenoma. Gastrointest. Endosc. 2000, 52, 27–32.
- Abraham, S.C.; Park, S.J.; Lee, J.H.; Mugartegui, L.; Wu, T.T. Genetic alterations in gastric adenomas of intestinal and foveolar phenotypes. Mod. Pathol. 2003, 16, 786–795.
- Borch, K.; Skarsgård, J.; Franzén, L.; Mårdh, S.; Rehfeld, J.F. Benign gastric polyps: Morphological and functional origin. Dig. Dis. Sci. 2003, 48, 1292–1297.
- Odze, R.D.; Marcial, M.A.; Antonioli, D. Gastric fundic gland polyps: A morphological study including mucin histochemistry, stereometry, and MIB-1 immunohistochemistry. Hum. Pathol. 1996, 27, 896–903.
- Zwick, A.; Munir, M.; Ryan, C.K.; Gian, J.; Burt, R.W.; Leppert, M.; Spirio, L.; Chey, W.Y. Gastric adenocarcinoma and dysplasia in fundic gland polyps of a patient with attenuated adenomatous polyposis coli. Gastroenterology 1997, 113, 659–663.
- Jalving, M.; Koornstra, J.J.; Götz, J.M.; van der Waaij, L.A.; de Jong, S.; Zwart, N.; Karrenbeld, A.; Kleibeuker, J.H. High-grade dysplasia in sporadic fundic gland polyps: A case report and review of the literature. Eur. J. Gastroenterol. Hepatol. 2003, 15, 1229–1233.
- Sekine, S.; Shibata, T.; Yamauchi, Y.; Nakanishi, Y.; Shimoda, T.; Sakamoto, M.; Hirohashi, S. Beta-catenin mutations in sporadic fundic gland polyps. Virchows Arch. 2002, 440, 381–386.
- Straub, S.F.; Drage, M.G.; Gonzalez, R.S. Comparison of dysplastic fundic gland polyps in patients with and without familial adenomatous polyposis. Histopathology 2018, 72, 1172–1179.
- Iwama, T.; Mishima, Y.; Utsunomiya, J. The impact of familial adenomatous polyposis on the tumorigenesis and mortality at the several organs. Its rational treatment. Ann. Surg. 1993, 217, 101–108.
- Mankaney, G.; Leone, P.; Cruise, M.; LaGuardia, L.; O’Malley, M.; Bhatt, A.; Church, J.; Burke, C.A. Gastric cancer in FAP: A concerning rise in incidence. Fam. Cancer 2017, 16, 371–376.
- Walton, S.J.; Frayling, I.M.; Clark, S.K.; Latchford, A. Gastric tumours in FAP. Fam. Cancer 2017, 16, 363–369.
- Li, J.; Woods, S.L.; Healey, S.; Beesley, J.; Chen, X.; Lee, J.S.; Sivakumaran, H.; Wayte, N.; Nones, K.; Waterfall, J.J.; et al. Point Mutations in Exon 1B of APC Reveal Gastric Adenocarcinoma and Proximal Polyposis of the Stomach as a Familial Adenomatous Polyposis Variant. Am. J. Hum. Genet. 2016, 98, 830–842.
- Worthley, D.L.; Phillips, K.D.; Wayte, N.; Schrader, K.A.; Healey, S.; Kaurah, P.; Shulkes, A.; Grimpen, F.; Clouston, A.; Moore, D.; et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): A new autosomal dominant syndrome. Gut 2012, 61, 774–779.
- De Boer, W.B.; Ee, H.; Kumarasinghe, M.P. Neoplastic Lesions of Gastric Adenocarcinoma and Proximal Polyposis Syndrome (GAPPS) Are Gastric Phenotype. Am. J. Surg. Pathol. 2018, 42, 1–8.
- Genta, R.M.; Schuler, C.M.; Robiou, C.I.; Lash, R.H. No association between gastric fundic gland polyps and gastrointestinal neoplasia in a study of over 100,000 patients. Clin. Gastroenterol. Hepatol. 2009, 7, 849–854.
- Dickey, W.; Kenny, B.D.; McConnell, J.B. Prevalence of fundic gland polyps in a western European population. J. Clin. Gastroenterol. 1996, 23, 73–75.
- Lloyd, I.E.; Kohlmann, W.K.; Gligorich, K.; Hall, A.; Lyon, E.; Downs-Kelly, E.; Samowitz, W.S.; Bronner, M.P. A Clinicopathologic Evaluation of Incidental Fundic Gland Polyps With Dysplasia: Implications for Clinical Management. Am. J. Gastroenterol. 2017, 112, 1094–1102.
- Fossmark, R.; Jianu, C.S.; Martinsen, T.C.; Qvigstad, G.; Syversen, U.; Waldum, H.L. Serum gastrin and chromogranin A levels in patients with fundic gland polyps caused by long-term proton-pump inhibition. Scand. J. Gastroenterol. 2008, 43, 20–24.
- Martin, F.C.; Chenevix-Trench, G.; Yeomans, N.D. Systematic review with meta-analysis: Fundic gland polyps and proton pump inhibitors. Aliment. Pharmacol. Ther. 2016, 44, 915–925.
- Jalving, M.; Koornstra, J.J.; Wesseling, J.; Boezen, H.M.; De Jong, S.; Kleibeuker, J.H. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment. Pharmacol. Ther. 2006, 24, 1341–1348.
- Watanabe, N.; Seno, H.; Nakajima, T.; Yazumi, S.; Miyamoto, S.; Matsumoto, S.; Itoh, T.; Kawanami, C.; Okazaki, K.; Chiba, T. Regression of fundic gland polyps following acquisition of Helicobacter pylori. Gut 2002, 51, 742–745.
- Kim, G.H. Proton Pump Inhibitor-Related Gastric Mucosal Changes. Gut Liver 2020, 15, 1976–2283.
- Kim, J.S.; Chae, H.S.; Kim, H.K.; Cho, Y.S.; Park, Y.W.; Son, H.S.; Han, S.W.; Choi, K.Y. Spontaneous resolution of multiple fundic gland polyps after cessation of treatment with omeprazole. Korean J. Gastroenterol. 2008, 51, 305–308.
- Hamada, K.; Takeuchi, Y.; Akasaka, T.; Iishi, H. Fundic Gland Polyposis Associated with Proton-Pump Inhibitor Use. Eur. J. Case Rep. Intern. Med. 2017, 4, 000607.
- Kazantsev, G.B.; Schwesinger, W.H.; Heim-Hall, J. Spontaneous resolution of multiple fundic gland polyps after cessation of treatment with lansoprazole and Nissen fundoplication: A case report. Gastrointest. Endosc. 2002, 55, 600–602.
- Fukuda, M.; Ishigaki, H.; Sugimoto, M.; Mukaisho, K.I.; Matsubara, A.; Ishida, H.; Moritani, S.; Itoh, Y.; Sugihara, H.; Andoh, A.; et al. Histological analysis of fundic gland polyps secondary to PPI therapy. Histopathology 2019, 75, 537–545.
- Shibukawa, N.; Wakahara, Y.; Ouchi, S.; Wakamatsu, S.; Kaneko, A. Synchronous Three Gastric Fundic Gland Polyps with Low-grade Dysplasia Treated with Endoscopic Mucosal Resection after Being Diagnosed to Be Tubular Adenocarcinoma Based on a Biopsy Specimen. Intern. Med. 2019, 58, 1871–1875.
- Martín Domínguez, V.; Díaz Méndez, A.; Santander, C.; García-Buey, L. Portal hypertensive polyps, a new entity? Rev. Esp. Enferm. Dig. 2016, 108, 279–280.
- Yasugi, K.; Haruma, K.; Kawanaka, M.; Suehiro, M.; Nakamura, J.; Urata, N.; Tanikawa, T.; Oka, T.; Monobe, Y.; Fujita, T.; et al. Disappearance of Gastric Hyperplastic Polyps after the Discontinuation of Proton Pump Inhibitor in a Patient with Liver Cirrhosis. Case Rep. Gastroenterol. 2021, 15, 202–209.
- Topal, F.; Akbulut, S.; Karahanlı, C.; Günay, S.; Sarıtaş Yüksel, E.; Topal, F.E. Portal Hypertensive Polyps as Gastroscopic Finding in Liver Cirrhosis. Gastroenterol. Res. Pract. 2020, 2020, 9058909.
- Seleem, W.M.; Hanafy, A.S. Management of a Portal Hypertensive Polyp: Case Report of a Rare Entity. Gastrointest. Tumors 2019, 6, 137–141.
- Andersson, T.; Olsson, R.; Regårdh, C.G.; Skånberg, I. Pharmacokinetics of [14C]omeprazole in patients with liver cirrhosis. Clin. Pharmacokinet. 1993, 24, 71–78.
