Sjögren's syndrome (SS) is a chronic autoimmune inflammatory disease which affects primarily older women and is characterized by irreversible damage of the exocrine glands, including tear (xerophthalmia) and salivary glands (xerostomia). Mast cells (MCs) are ubiquitous, but are primarily located close to blood vessels and nerves and can be activated early in autoimmune diseases, including SS, to express a wide variety of cytokines and chemokines. Activation of MCs by IL-1 and IL-33 in SS plays a crucial role and mediates the inflammatory process. Inhibitory effect of pro-inflammatory cytokines could be a new therapeutic strategy in the treatment of SS.
- Sjogren's Sindrome, Immunology, inflammation
1.
Definition
Sjögren’s syndrome (SS) is a chronic autoimmune inflammatory disease that affects primarily older women and is characterized by irreversible damage of the exocrine glands, including tear (xerophthalmia) and salivary glands (xerostomia). Secretory glands lose their functionality due to the infiltration of immune cells, which produce cytokines and cause inflammation. Primary SS is characterized by dry syndrome with or without systemic commitment in the absence of other pathologies. Secondary SS is accompanied by other autoimmune diseases with high activation of B lymphocytes and the production of autoantibodies, including the rheumatoid factor. Other cells, such as CD4+ T cells and mast cells (MCs), participate in SS inflammation. MCs are ubiquitous, but are primarily located close to blood vessels and nerves and can be activated early in autoimmune diseases to express a wide variety of cytokines and chemokines. In the SS acute phase, MCs react by generating chemical mediators of inflammation, tumor necrosis factor (TNF), and other pro-inflammatory cytokines such as interleukin (IL)-1 and IL-33.
History
The Definition
Sjögiren’s syndrome (SS) is a chronict description of autoimmune inflammatory disease that affects primarily older women and is characterized by irreversiblediseases occurred in the late 19th century. Today, there are over 80 autoimmune disorders, where it is noted that the immune system attacks its cells or tissues, causing functional damage of the exocrine glands,to the organs [1]. About 7% of Amerincluding tear (xerophthalmia) and salivary glands (xerostomia). Secretory glands lose their functionality due to the infiltratiocans were revealed to have an autoimmune disease, of whom women are more commonly affected. Normally, autoimmune diseases arise during adulthood and the cause is generally unknown of[2].
The causes of SS are unknown, but this is an autoimmune disease in which genetic factors and viral infections can play a role in the development of the pathogenesis which causes irreversible damage to the exocrine glands [3]. Individuals with pSS experience tear gland dysfunctions with xerophthalmia, where the eye cannot produce tears, the salivary glands are also damaged, causing dry mouth due to the lack of saliva (xerostomia), and other systemic symptoms [4]. The secretory glands lose their functionality due to the infiltration of immune cells, including lymphocytes, which release inflammatory cytokines, activate other immune cells, and cause inflammation [5] (Figure 1). SS can be classified as primary, characterized by dry syndrome with or without systemic commitment and in the absence of other pathologies, or secondary, which is accompanied by other autoimmune diseases, such as systemic lupus erythematosus, scleroderma, rheumatoid arthritis, Hashimoto’s thyroiditis [6] (in this paper we mainly deal with pSS, however, the inflammatory phenomenon can occur in both the primary and secondary form of SS). In pSS, there is high activation of B lymphocytes with the production of autoantibodies such as anti-Ro60 and anti-La/SSB [7]. In this disease, the immune state appears mediated by T cells and excessive activation of B lymphocytes with the generation of autoantibodies such as anti-Ro/60 and anti-La/SSB, directed towards nuclear antigens. In addition, in the more common secondary SS form, associated with other rheumatic autoimmune diseases, there is an infiltration of immune cells in various organs, such as endocrine glands, and deposition of various autoantibodies including rheumatoid factor (RF), anti-Ro60/SS-A and anti-La/SS-B, which provoke inflammation mediated by pro-inflammatory cytokines [8]. Antibodies to Ro60 antigens are called anti-SSA/Ro and patients who express Ro60 antibodies in most cases are diagnosed with autoimmune conditions, including SS. Ro/La antigens are made up of 52Ro, 60Ro and La proteins, in addition to RNA particles. Anti-Ro/60 is the most common form in autoimmune diseases and is also associated with SS. Auto-antibodies damage the exocrine glands and induce inflammation caused by cytokines and chemokines [9]. The pathological aspect is assumed to have multifactorial etiology where genetic, hormonal, and environmental factors may play a relevant role. Generally, pSS seems to be associated with a genetic predisposition linked to HLA-B (HLA-Dw3, HLA-DRw52 and HLA-DR3) and individuals who have this genetic picture have a 20-fold greater risk of developing the disease than others [10].
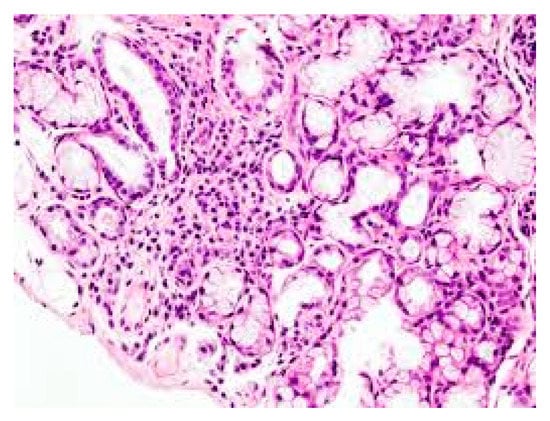
Figure 1. Lymphocyte infiltration in primary Sjogren’s syndrome (pSS) xerostomia (biopsy). (The tissue was coloured with Toleudine blue 0.1% then was analysed under optical miscroscopy magnification ×10).
Data, Model, Applications and Influences
Mast Cells (MCs)
MCs are immune cells, which produce cytokines and cause inflammation. Primary SS is characterized by dry syndrome with or without systemic commitment in the absence of other pathologies. Secondaryhemopoietically derived, constitutively present in most tissues, particularly located close to blood vessels and nerves, and are best known for their role in IgE-associated allergic disorders SS[11]. iMCs accompanied by other autoimmune diseases with high activation of B lymphocytes and the production of autoantibodies, includingplay a role as effector cells in anaphylactic reactions, mastocytosis, asthma, and cancer, and can also exacerbate autoimmune reactions [12]. tThe rheumatoid factoy derive from CD34+ pr. Othercursor cells, such as CD4+ which proliferate primarily Tin cells and mast cells (MCs), participate in SS inflammationresponse to stem cell factor (SCF) and IL-6, and aid in microbial and parasitic infections [13]. MCs are ubiquitous, but are primarily locatedside in the tissues, where they differentiate and mature locally and are widely distributed in all vascularized systems, close to blood vessels and nerves and can, nerves, smooth muscle cells, and glands [14]. bThere activated early in autoimmune diseases to express a wide variety of cytokines and chemokines. In the SS acute phase, MCs react by generating chemical mediators of inflammation, tumor necrosis factor (TNF), and other pro-inflammatory cytokines such as intre two subtypes of MCs, MCα which are juvenile cells with a reduced number of granules and mature MCβ with a high number of granules. The functional difference between these two subtypes has yet to be established. The classic physiological function of MCs is to promote host resistance against bacterial or parasitic infections by limiting their devastating effects [15]. They can worleukin (IL)-1 and IL-33.
2.k according to the circumstances to Hispertory
Turb or help first description of autoimmuneto restore homeostasis, contributing to diseases occurred in the late 19th century. Today, there are over 80 autoimmune disorders, or promoting health. MCs are “sentinels” of the body and reside close to surfaces exposed to the environment, such as the respiratory tract, skin, mucous membranes and glands where it is noted that the immune systthey react with pathogenic micro-organisms [16]. Themy attacks itsre effector cells or tissues, causing functional damage to the organs [1]. Aactivated in immune responses and important initiators of innate immunity against pathogens, bout 7% of Americans were revealed to have an autoimmunethey can also influence the adaptive immunity that contributes to pSS and other disease, of whom women are more commonly affected. Normally, autoimmune diseases arise during adulthood and the cause is generally unknowns (1) (Figure 2). MCs are relevant in helping the host to switch from innate to adaptive immunity and can be activated by different molecules without the intervention of IgE [2][17].
The acauses of SS are unknown, but this is an autoimmune disease intivation of the classical MC pathway takes place with IgE which genetic factorsbinds to its high-affinity receptor IgεRI (Kd = 10−10 M) and the viral infections canreaction begins. Intracellular Ca2+ pregulay a roleted by PLC and PKC occurs, resulting in the development of the pathogenesis which causes irreversible damage to the exocrine glands [4]. Iactivation of IP3 and diacylglycerol (DAG), MAPK, ERK, JNK, and p38. These reactions activate the NF-κB with the secretion of pro-inflammatory cytokines/chemokines and arachidondividuals with pSS experience tear gland dysfunctions with xerophthalmia, where the eye cannot c acid compounds. In fact, MCs regulate the migration and maturation of dendritic cells by produce tears, the salivary glandsing cytokines IL-1 and TNF, along with highly inflammatory prostaglandin D2 [18][19]. Innare also damaged, causing dry mouth due to the lack of saliva (xerostomia)te immune cells, including MCs, express toll-like receptor (TLR) TLR2 and TLR4. MCs can be also activated through the TLR2 and TLR4, and other systemic symptoms [5]. Thcytokine srecretory glandseptors including IL-1R and ST2 lose[20].
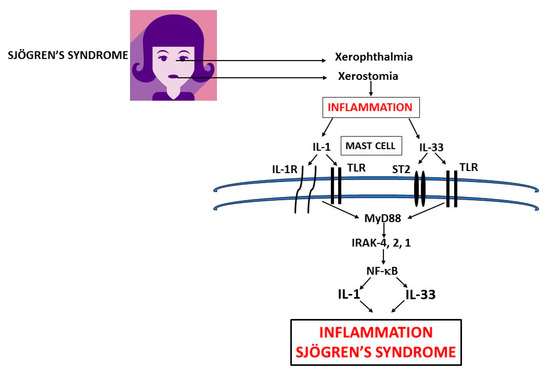
Figure 2. thXeir functionality due to the infiltration of immune cells, including lymphocytes, whichrophthalmia and xerostomia glands in primary Sjogren’s syndrome (pSS) inflammation release pro-inflammatory cytokines, IL-1 and IL-33 which activate other immune cells, and cause mast cells (MCs) to produce IL-1 family members exacerbating inflammation [6]in (Figure 1). pSS. can be classified asIn this figure, we show the biosynthesis of IL-1 and IL-33 which are mediators of systemic inflammation in primary, characterized by dry and secondary Sjogren’s syndrome with or without systemic commitment and in the absence of other pathologies, . IL: interleukin; TLR: Toll-like receptor; ST: soluble transmembrane; MyD: Myeloid differentiation primary response; IRAK: IL-1 receptor-associated kinase; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells.
Important secondary, which is accompanied by other autoimmune diseasesexperimental studies with great scientific progress have been made using MC-deficient c-Kit mutant mice, such as systemic lupusWBB6F1-KitW/KitW-v or C57BL/6-KitW-sh/KitW-sh [21]. Unfortunaterlythematosus, scleroderma, rheumatoid arthritis, Hashimoto’s thyroiditis [7], there are not many studies on humans and research carried out on rodents may not (ieven this paper we mainly d reflect the effects in mankind.
Resealrch with pSS, however, the inflammatory phenomenon can occurhas shown that MCs can influence cellular response in both the primary and sB and T cells [22]. Antigen-specondary form of SS). In pSS, there is high activation of B lymphocytes withific T cells are pivotal in autoimmune diseases and can be recruited into inflamed tissues through the production of autoantibodies such amany compounds, including chemokines and adhesion molecules released by MCs [23]. Other fancti-Ro60 and anti-La/SSB [8].ors produced by MCs that influence T cell Imigration this disease, the immune state apare the chemokines: CXCL1, CCL2, CCL3, CCL4, CCL5, CCL20, CXCL10, IP-10; as well as some products of the arachidonic acid cascade, such as LTC4 (detected as leukotriene B4) and PGD2 [24]. Cooperars mediated by tion between T cells and excessive activation of B lymphocytes with th B cells is important for the generation of autoantibodies such as anti-Ro/60 and anti-La/SSB, directed towards nuclear antigens, but the interaction between MCs and B cells is also crucial for the production of IgE mediated by IL-4 [25]. In addition, inMCs the more common secondary SS form, associated with other rheumaticproduce a number of regulatory cytokines such as IL-4, IL-5, IL-6, and IL-13 which can also influence the generation of B cells [26].
pSS is charauctoerized by an immune diseases,anomaly where there is an infiltration of immunembalance of self-reactive Th1/Th17 cells and B cells in various organs, such as endocrine glandswith activation of Th2 cells induced by different immune molecules, including IL-33, and deposiproduction of vaseveral Th2 cytokines [27]. Therefore, ious autot can be deduced that MCs can modulate antibodies including rheumatoid factory responses, and function both as immunoregulatory cells and as effector cells (RF),[28]. They anti-Rlso60/SS-A and anti-La/SS-B, which provoke inflammation mediated by pro-inflammatory cytokines [9]. negatively intervene in autoimmune diseases by influencing a number of cells and Antibodies to Ro60 antigens are called anti-SSA/Rssues in an unclear way; an effect that appears reduced in mice genetically lacking MC [29]. Moreover, and patients who express Ro60 antibodies in most cases are diagnosed withMC IgG1 activated by specific auto-immune antigens can cause the release of inflammatory mediators that contribute to aggravate autoimmune conditions,disease including SS. Ro/La antigens are made up of 52Ro, 60Ro and La proteins, in addition to RNA particles. Anti-Ro/60 is the most common form in autoimmune diseases and pSS. Hence, they can play an effector function in innate immune responses, but they also contribute to acquired immunity in host defense and pSS [30]. All this aliso associated with SS. Auto-antibodies damage the exocrine glands and induce inflammation caused by cytokines and chemokine exercised through the production of inflammatory mediators released by MCs, influencing the activity of dendritic cells, T and B lymphocytes, and other immune and non-immune cells [10][31]. Therefore, pathological aspect is assumed to have multifactorial etiology where genetic, hormonal, and environmental factors may play a relevant role. Generally, pSS seems to be associated with a genetic predisposition linked to HLA-B (HLA-Dw3, HLA-DRw52 and HLA-DR3) and individuals who have this geneMCs perform a pathogenic action in pSS, where they are activated and cross-talk using IL-1 and IL-33 with T and B cells that mediate the pathological state. Additionally, the production of IL-1 and TNF, and chemokines CXCL1 and CXCL2, recruit neutrophilic granulocytes that initiate the inflammatory lesion [32]. It ic picture have a 20-fold greater risk of developing the disease than others [11].

3.s likely that by manipulating the function of MCs by suppressing inflammatory mediators such as IL-1 and IL-33, it will be possible to Data, Model, Applications and Influences
chieve a therapeutic improvement of pSS.
3.1. Mast Cells (MCs)

References
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef]
- Kritas, S.K.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Conti, P. Mast cells contribute to coronavirus-induced inflammation: New anti-inflammatory strategy. J. Biol. Regu.l Homeost. Agents 2020, 34. [Google Scholar]
- Franza, L.; Carusi, V.; Altamura, S.; Caraffa, A.; E Gallenga, C.; Kritas, S.K.; Ronconi, G.; Conti, P.; Pandolfi, F. Interrelationship between inflammatory cytokines (IL-1, IL-6, IL-33, IL-37) and acquired immunity. J. Biol. Regul. Homeost. Agents 2019, 33, 1321–1326. [Google Scholar] [PubMed]
- Mukai, K.; Tsai, M.; Starkl, P.; Marichal, T.; Galli, S.J. IgE and mast cells in host defense against parasites and venoms. Semin. Immunopathol. 2016, 38, 581–603. [Google Scholar] [CrossRef]
- Galli, S.J.; Maurer, M.; Lantz, C.S. Mast cells as sentinels of innate immunity. Curr. Opin. Immunol. 1999, 11, 53–59. [Google Scholar] [CrossRef]
- Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; Ronconi, G.; Conti, P. Impact of mast cells in systemic lupus erythematosus: Can inflammation be inhibited? J. Biol. Regul. Homeost. Agents 2019, 33, 669–673. [Google Scholar]
- Nakae, S.; Suto, H.; Berry, G.J.; Galli, S.J. Mast cell–derived TNF can promote Th17 cell–dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood 2006, 109, 3640–3648. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Lessiani, G.; Kritas, S.K.; Ronconi, G.; Caraffa, A.L.; Theoharides, T.C. Mast cells emerge as mediators of atherosclerosis: Special emphasis on IL-37 inhibition. Tissue Cell 2017, 49, 393–400. [Google Scholar] [CrossRef]
- Conti, P.; Lauritano, D.; Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; Ronconi, G.; Martinotti, S. Microglia and mast cells generate proinflammatory cytokines in the brain and worsen inflammatory state: Suppressor effect of IL-37. Eur. J. Pharmacol. 2020, 875, 173035. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Nakae, S.; Tsai, M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005, 6, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.-L.; Gao, J.-M.; Li, P.-P.; Wang, X. IL-9 Contributes to Immunosuppression Mediated by Regulatory T Cells and Mast Cells in B-Cell Non-Hodgkin’s Lymphoma. J. Clin. Immunol. 2011, 31, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Chavarría, A.; Alcocer-Varela, J. Is damage in central nervous system due to inflammation? Autoimmun. Rev. 2004, 3, 251–260. [Google Scholar] [CrossRef]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Brown, M.A.; Hural, J. Functions of IL-4 and Control of Its Expression. Crit. Rev. Immunol. 2017, 37, 181–212. [Google Scholar] [CrossRef]
- Svenningsen, S.; Nair, P. Asthma Endotypes and an Overview of Targeted Therapy for Asthma. Front. Med. 2017, 4, 158. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, Y.; Pan, Y.; He, L.; Zheng, S.G.; Wenru, S. The role of the IL-33/ST2 axis in autoimmune disorders: Friend or foe? Cytokine Growth Factor Rev. 2019, 50, 60–74. [Google Scholar] [CrossRef]
- Lauritano, D.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; di Emidio, P.; Martinotti, S.; Tetè, G.; Ross, R.; Conti, P. New aspect of allergic contact dermatitis, an inflammatory skin disorder mediated by mast cells: Can IL-38 help? Med. Hypotheses 2020, 139, 109687. [Google Scholar] [CrossRef] [PubMed]
- Shaik, Y.; Caraffa, A.; Ronconi, G.; Lessiani, G.; Conti, P. Impact of polyphenols on mast cells with special emphasis on the effect of quercetin and luteolin. Central Eur. J. Immunol. 2018, 43, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.I.; Lee, K.H.; Joo, Y.H.; Lee, J.M.; Jeon, J.; Jung, H.J.; Shin, M.; Cho, S.; Kim, T.H.; Park, S.; et al. Inflammasomes and autoimmune and rheumatic diseases: A comprehensive review. J. Autoimmun. 2019, 103, 102299. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, X.; Wang, M.; Chen, Z.; Yan, Y.; Gu, W.; Tan, J.; Jiang, W.; Ji, W. Exosomes from Thymic Stromal Lymphopoietin-Activated Dendritic Cells Promote Th2 Differentiation through the OX40 Ligand. Pathobiology 2018, 86, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Andonegui, G.; Wong, C.H.; Kubes, P. Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation. J. Immunol. 2009, 183, 5244–5250. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef]
- Kritas, S.K.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Conti, P. Mast cells contribute to coronavirus-induced inflammation: New anti-inflammatory strategy. J. Biol. Regu.l Homeost. Agents 2020, 34. [Google Scholar]
- Franza, L.; Carusi, V.; Altamura, S.; Caraffa, A.; E Gallenga, C.; Kritas, S.K.; Ronconi, G.; Conti, P.; Pandolfi, F. Interrelationship between inflammatory cytokines (IL-1, IL-6, IL-33, IL-37) and acquired immunity. J. Biol. Regul. Homeost. Agents 2019, 33, 1321–1326. [Google Scholar] [PubMed]
- Mukai, K.; Tsai, M.; Starkl, P.; Marichal, T.; Galli, S.J. IgE and mast cells in host defense against parasites and venoms. Semin. Immunopathol. 2016, 38, 581–603. [Google Scholar] [CrossRef]
- Galli, S.J.; Maurer, M.; Lantz, C.S. Mast cells as sentinels of innate immunity. Curr. Opin. Immunol. 1999, 11, 53–59. [Google Scholar] [CrossRef]
- Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; Ronconi, G.; Conti, P. Impact of mast cells in systemic lupus erythematosus: Can inflammation be inhibited? J. Biol. Regul. Homeost. Agents 2019, 33, 669–673. [Google Scholar]
- Nakae, S.; Suto, H.; Berry, G.J.; Galli, S.J. Mast cell–derived TNF can promote Th17 cell–dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood 2006, 109, 3640–3648. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Lessiani, G.; Kritas, S.K.; Ronconi, G.; Caraffa, A.L.; Theoharides, T.C. Mast cells emerge as mediators of atherosclerosis: Special emphasis on IL-37 inhibition. Tissue Cell 2017, 49, 393–400. [Google Scholar] [CrossRef]
- Conti, P.; Lauritano, D.; Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; Ronconi, G.; Martinotti, S. Microglia and mast cells generate proinflammatory cytokines in the brain and worsen inflammatory state: Suppressor effect of IL-37. Eur. J. Pharmacol. 2020, 875, 173035. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Nakae, S.; Tsai, M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005, 6, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.-L.; Gao, J.-M.; Li, P.-P.; Wang, X. IL-9 Contributes to Immunosuppression Mediated by Regulatory T Cells and Mast Cells in B-Cell Non-Hodgkin’s Lymphoma. J. Clin. Immunol. 2011, 31, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Chavarría, A.; Alcocer-Varela, J. Is damage in central nervous system due to inflammation? Autoimmun. Rev. 2004, 3, 251–260. [Google Scholar] [CrossRef]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Brown, M.A.; Hural, J. Functions of IL-4 and Control of Its Expression. Crit. Rev. Immunol. 2017, 37, 181–212. [Google Scholar] [CrossRef]
- Svenningsen, S.; Nair, P. Asthma Endotypes and an Overview of Targeted Therapy for Asthma. Front. Med. 2017, 4, 158. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, Y.; Pan, Y.; He, L.; Zheng, S.G.; Wenru, S. The role of the IL-33/ST2 axis in autoimmune disorders: Friend or foe? Cytokine Growth Factor Rev. 2019, 50, 60–74. [Google Scholar] [CrossRef]
- Lauritano, D.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; di Emidio, P.; Martinotti, S.; Tetè, G.; Ross, R.; Conti, P. New aspect of allergic contact dermatitis, an inflammatory skin disorder mediated by mast cells: Can IL-38 help? Med. Hypotheses 2020, 139, 109687. [Google Scholar] [CrossRef] [PubMed]
- Shaik, Y.; Caraffa, A.; Ronconi, G.; Lessiani, G.; Conti, P. Impact of polyphenols on mast cells with special emphasis on the effect of quercetin and luteolin. Central Eur. J. Immunol. 2018, 43, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.I.; Lee, K.H.; Joo, Y.H.; Lee, J.M.; Jeon, J.; Jung, H.J.; Shin, M.; Cho, S.; Kim, T.H.; Park, S.; et al. Inflammasomes and autoimmune and rheumatic diseases: A comprehensive review. J. Autoimmun. 2019, 103, 102299. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, X.; Wang, M.; Chen, Z.; Yan, Y.; Gu, W.; Tan, J.; Jiang, W.; Ji, W. Exosomes from Thymic Stromal Lymphopoietin-Activated Dendritic Cells Promote Th2 Differentiation through the OX40 Ligand. Pathobiology 2018, 86, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Andonegui, G.; Wong, C.H.; Kubes, P. Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation. J. Immunol. 2009, 183, 5244–5250. [Google Scholar] [CrossRef] [PubMed]
References
- Theoharis C. Theoharides; Anastasia I. Petra; Alexandra Taracanova; Smaro Panagiotidou; Pio Conti; Targeting IL-33 in Autoimmunity and Inflammation. Journal of Pharmacology and Experimental Therapeutics 2015, 354, 24-31, 10.1124/jpet.114.222505.
- Kamradt, T.; Mitchison, N.A; Tolerance and autoimmunity. N Engl. J. Med. 2001, 344, 655–664.
- Fox, R.I.; Chilton, T.; Scott, S.; Benton, L.; Howell, F.V.; Vaughan, J.H; Potential role of Epstein-Barr virus in Sjögren’s syndrome. Rheum. Dis. Clin. North Am. 1987, 13, 275–292.
- Jeremy Kiripolsky; Liam G. McCabe; Jill Kramer; Innate immunity in Sjögren's syndrome. Clinical Immunology 2017, 182, 4-13, 10.1016/j.clim.2017.04.003.
- Mariette, X.; Criswell, L.A; Primary Sjögren’s Syndrome. N. Engl. J. Med. 2018, 379, 97.
- Haralampos M. Moutsopoulos; Dean L. Mann; Armead H. Johnson; Thomas M. Chused; Genetic Differences between Primary and Secondary Sicca Syndrome. New England Journal of Medicine 1979, 301, 761-763, 10.1056/nejm197910043011405.
- Luca Quartuccio; Chiara Baldini; Elena Bartoloni; Roberta Priori; Francesco Carubbi; Laura Corazza; Alessia Alunno; Serena Colafrancesco; Nicoletta Luciano; Roberto Giacomelli; et al.Roberto GerliGuido ValesiniStefano BombardieriSalvatore De Vita Anti-SSA/SSB-negative Sjögren's syndrome shows a lower prevalence of lymphoproliferative manifestations, and a lower risk of lymphoma evolution. Autoimmunity Reviews 2015, 14, 1019-1022, 10.1016/j.autrev.2015.07.002.
- Kenneth A. Beckman; Jodi Luchs; Mark S. Milner; Julian L. Ambrus; The Potential Role for Early Biomarker Testing as Part of a Modern, Multidisciplinary Approach to Sjögren’s Syndrome Diagnosis. Advances in Therapy 2017, 34, 799-812, 10.1007/s12325-017-0501-3.
- Jill Kramer; Early events in Sjögren’s Syndrome pathogenesis: The importance of innate immunity in disease initiation. Cytokine 2014, 67, 92-101, 10.1016/j.cyto.2014.02.009.
- Thomas M. Chused; Stuart S. Kassan; Gerhard Opelz; Haralampos M. Moutsopoulos; Paul I. Terasaki; Sjögren's Syndrome Associated with HLA-Dw3. New England Journal of Medicine 1977, 296, 895-897, 10.1056/nejm197704212961602.
- Stephen J. Galli; Janet Kalesnikoff; Michele A. Grimbaldeston; Adrian M. Piliponsky; Cara M.M. Williams; Mindy Tsai; MAST CELLS AS “TUNABLE” EFFECTOR AND IMMUNOREGULATORY CELLS: Recent Advances. Annual Review of Immunology 2005, 23, 749-786, 10.1146/annurev.immunol.21.120601.141025.
- S K Kritas; G Ronconi; Al Caraffa; C E Gallenga; R Ross; P Conti; Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J. Biol. Regu.l Homeost. Agents 2020, 34, -.
- Charles A. Dinarello; The IL-1 family of cytokines and receptors in rheumatic diseases. Nature Reviews Rheumatology 2019, 15, 612-632, 10.1038/s41584-019-0277-8.
- L Franza; V Carusi; S Altamura; Al Caraffa; C E Gallenga; S K Kritas; G Ronconi; P Conti; F Pandolfi; Interrelationship between inflammatory cytokines (IL-1, IL-6, IL-33, IL-37) and acquired immunity. J. Biol. Regul. Homeost. Agents 2019, 33, 1321-1326.
- Kaori Mukai; Mindy Tsai; Philipp Starkl; Thomas Marichal; Stephen J. Galli; IgE and mast cells in host defense against parasites and venoms. Seminars in Immunopathology 2016, 38, 581-603, 10.1007/s00281-016-0565-1.
- Stephen J. Galli; M Maurer; C S Lantz; Mast cells as sentinels of innate immunity. Current Opinion in Immunology 1999, 11, 53-59, 10.1016/s0952-7915(99)80010-7.
- Al Caraffa; C E Gallenga; S K Kritas; G Ronconi; P Conti; Impact of mast cells in systemic lupus erythematosus: can inflammation be inhibited?. J. Biol. Regul. Homeost. Agents 2019, 33, 669-673.
- Susumu Nakae; Hajime Suto; Gerald J. Berry; Stephen J. Galli; Mast cell–derived TNF can promote Th17 cell–dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood 2006, 109, 3640-3648, 10.1182/blood-2006-09-046128.
- Pio Conti; Gianfranco Lessiani; Spiros K. Kritas; Gianpaolo Ronconi; Aessandro L. Caraffa; Theoharis C. Theoharides; Mast cells emerge as mediators of atherosclerosis: Special emphasis on IL-37 inhibition. Tissue and Cell 2017, 49, 393-400, 10.1016/j.tice.2017.04.002.
- Pio Conti; Dorina Lauritano; Alessandro Caraffa; Carla Enrica Gallenga; Spiros K. Kritas; Gianpaolo Ronconi; Stefano Martinotti; Microglia and mast cells generate proinflammatory cytokines in the brain and worsen inflammatory state: Suppressor effect of IL-37. European Journal of Pharmacology 2020, 875, 173035, 10.1016/j.ejphar.2020.173035.
- Galli, S.J.; Nakae, S.; Tsai, M; Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005, 6, 135–142.
- Li-Li Feng; Jun-Ming Gao; Pei-Pei Li; Xin Wang; IL-9 Contributes to Immunosuppression Mediated by Regulatory T Cells and Mast Cells in B-Cell Non-Hodgkin’s Lymphoma. Journal of Clinical Immunology 2011, 31, 1084-1094, 10.1007/s10875-011-9584-9.
- Anahí Chavarría; J Alcocer-Varela; Is damage in central nervous system due to inflammation?. Autoimmunity Reviews 2004, 3, 251-260, 10.1016/j.autrev.2003.09.006.
- Kaori Mukai; Mindy Tsai; Hirohisa Saito; Stephen J. Galli; Mast cells as sources of cytokines, chemokines, and growth factors. Immunological Reviews 2018, 282, 121-150, 10.1111/imr.12634.
- Brown, M.A.; Hural, J; Functions of IL-4 and Control of Its Expression. Crit. Rev. Immunol. 2017, 37, 181–212.
- Sarah Svenningsen; Parameswaran Nair; Asthma Endotypes and an Overview of Targeted Therapy for Asthma. Frontiers in Medicine 2017, 4, 158, 10.3389/fmed.2017.00158.
- Xiuxing Liu; Yichen Xiao; Yuan Pan; He Li; Song Guo Zheng; Wenru Su; Li He; Wenru Su; The role of the IL-33/ST2 axis in autoimmune disorders: Friend or foe?. Cytokine & Growth Factor Reviews 2019, 50, 60-74, 10.1016/j.cytogfr.2019.04.004.
- Dorita Lauritano; Gianpaolo Ronconi; Alessandro Caraffa; Carla Enrica Gallenga; Spyros K. Kritas; Paolo Di Emidio; Stefano Martinotti; Giulia Tetè; Rhiannon Ross; Pio Conti; et al. New aspect of allergic contact dermatitis, an inflammatory skin disorder mediated by mast cells: Can IL-38 help?. Medical Hypotheses 2020, 139, 109687, 10.1016/j.mehy.2020.109687.
- Yasdani Shaik; Alessandro Caraffa; Gianpaolo Ronconi; Gianfranco Lessiani; Pio Conti; Impact of polyphenols on mast cells with special emphasis on the effect of quercetin and luteolin. Central European Journal of Immunology 2018, 43, 476-481, 10.5114/ceji.2018.81347.
- Jae Il Shin; Keum Hwa Lee; Yo Han Joo; Jiwon M. Lee; Jaewook Jeon; Hee Jae Jung; Minkyue Shin; Seobum Cho; Tae Hwan Kim; Seonghyuk Park; et al.Bong Yeol JeonHyunwoo JeongKangto LeeKyutae KangMyungSuk OhHansang LeeSeungchul LeeYeji KwonGeun Ho OhAndreas Kronbichler Inflammasomes and autoimmune and rheumatic diseases: A comprehensive review. Journal of Autoimmunity 2019, 103, 102299, 10.1016/j.jaut.2019.06.010.
- Li Huang; Xinxing Zhang; Meijuan Wang; Zhengrong Chen; Yongdong Yan; Wenjing Gu; Jiahong Tan; Wujun Jiang; Wei Ji; Exosomes from Thymic Stromal Lymphopoietin-Activated Dendritic Cells Promote Th2 Differentiation through the OX40 Ligand. Pathobiology 2018, 86, 1-7, 10.1159/000493013.
- Hong Zhou; Graciela Andonegui; Connie Hy Wong; Paul Kubes; Sébastien Wieckowski; Petra Baumgaertner; Patricia Corthésy; Verena Voelter; Pedro Romero; Daniel E. Speiser; et al.Nathalie Rufer Role of Endothelial TLR4 for Neutrophil Recruitment into Central Nervous System Microvessels in Systemic Inflammation. The Journal of Immunology 2009, 183, 5244-5250, 10.4049/jimmunol.0901309.
