Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Irene Gracia-Rubio.
The scavenger receptor B class type 1 (SR-B1) was identified as the high-affinity HDL receptor, which facilitates the selective uptake of cholesterol ester (CE) into the liver via HDL and is also implicated in the plasma clearance of LDL, very low-density lipoprotein (VLDL) and lipoprotein(a) (Lp(a)). Thus, SR-B1 is a multifunctional receptor that plays a main role in the metabolism of different lipoproteins.
- Scavenger receptor B class 1
- cardiovascular disease
- mice and human genetic studies
- high-density lipoprotein
- low-density lipoprotein
1. Introduction
Cardiovascular disease (CVD) remains the primary cause of mortality and morbidity worldwide [1]. The principal risk factor for developing CVD is relatively high plasma level of low-density lipoprotein cholesterol (LDLc) [2,3,4][2][3][4]. Numerous epidemiological and clinical investigations have revealed that plasma level of high-density lipoprotein cholesterol (HDLc) correlates inversely with the risk of CVD [5,6][5][6]. This association has been described by anti-atherogenic capacities of HDL, comprising its role in reverse cholesterol transport (RCT), in which cholesterol from peripheral tissues is transferred to the liver for excretion in bile and its ability to receive cholesterol from macrophages in the artery wall [7,8][7][8]. However, Mendelian randomization studies [9,10][9][10] and pharmacological interventional studies [11,12][11][12] do not support the concept that HDLc directly reduces the risk of CVD [8]. In addition, a retrospective analysis of large epidemiological studies showed that high HDLc concentration is associated with higher risk for CVD [13,14][13][14]. These results support the hypothesis that HDL metabolism and functionality is more important than HDLc levels for CVD risk prediction [14]. Acton et al. identified the scavenger receptor B class 1 (SR-B1) as a high-affinity HDL receptor, which facilitates the selective uptake of cholesterol esters (CE) in HDL into the liver [15]. This receptor is also implicated in the plasma clearance of LDL, very low-density lipoprotein (VLDL) and lipoprotein(a) (Lp(a)), lipoproteins with pro-atherogenic properties [16,17,18,19][16][17][18][19]. Therefore, SR-B1 is involved in cholesterol homeostasis, lipoprotein metabolism and atherosclerosis [4]. In addition, SR-B1 plays a relevant role in HDL-mediated cellular signaling [20], and might play a crucial role in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) [21], since this receptor is linked to dyslipidemia [22].
2. SR-B1 in Lipoprotein Metabolism
SR-B1 is mainly identified for promoting selective uptake of CE from HDL or other lipoproteins to cells by a non-endocytic process [23,24,25,26,27,28][23][24][25][26][27][28]. Moreover, SR-B1 mediates selective hepatic uptake of HDL-CE, free cholesterol (FC), triglycerides (TG), and phospholipids by a three step mechanism [24,25,26,27][24][25][26][27]. First, cholesterol-rich donor lipoprotein particles could bind to the extracellular loop domain of the receptor. Then, SR-B1 could promote the transfer of CE from the lipoprotein particles to the plasma membrane, and finally, the cholesterol poor lipoprotein particles could release back into the circulation [3,23,24][3][23][24]. Furthermore, SR-B1 requires oligomerization to promote selective lipid uptake, but not HDL binding [26,27,28,29,30,31][26][27][28][29][30][31]. Although the mechanism by which SR-B1 facilitates the transfer of CE to the plasma membrane is not fully understood, a model has been proposed in which a hydrophobic tunnel is formed by the extracellular domain of the receptor between lipoprotein particles and the cell membrane through which CE diffuse in a concentration gradient manner [27,32][27][32]. The recent publication of the high-resolution crystal structure of the extracellular domain of LIMP-2, a homologue of SR-B1, supports the validity of this mechanism [33].
In addition to selective CE uptake, SR-B1 also facilitates the efflux of free cholesterol between cells and lipoproteins [34,35][34][35]. Briefly, this mechanism carried out by HDL is known as RCT and consists of the transport of cholesterol via HDL from peripheral tissues such as macrophages or endothelial cells to the liver for cholesterol excretion, bile acid production or steroid hormone synthesis in steroidogenic organs [26,36][26][36]. Apart from SR-B1, two more receptors are involved in this process: ATP-binding cassette A1 (ABCA1), that mediates unidirectional efflux of cholesterol and phospholipids to apolipoprotein (apo) A-I and apo E [27[27][37],37], and ATP-binding cassette G1 (ABCG1), that promotes unidirectional efflux of cholesterol to nascent HDL particles [27,38][27][38].
3. SR-B1, an Important Participant in the Development of Cardiovascular Disease
SR-B1 has been involved in the progression of atherosclerosis [4]. SR-B1, via HDL, contributes to the transport of cholesterol from macrophages through cholesterol efflux to the liver, and is also implicated in reducing inflammation and oxidation [3,4,27][3][4][27]. SR-B1 interaction with HDL modulates macrophage inflammation through activation of Akt and decreased activation of nuclear factor-κB (NF-κB), promoting the release of anti-inflammatory cytokines, including interleukin 10 and transforming growth factor-beta (TGF-ß) [26,39][26][39]. In the endothelial cells, SR-B1 inhibits inflammation via endothelial nitric oxide synthase (eNOS) activation and expression of the antioxidant enzyme, 3-beta-hydroxysteroid-delta 24-reductase (DHCR24) [4,40][4][40]. HDL and apo A-I also reduce oxidative modification of apo B containing lipoproteins [4]. Furthermore, SR-B1 in macrophages and endothelial cells could suppress the progression of atherosclerosis by modifying cholesterol trafficking and reducing atherosclerotic lesion through limiting foam cell formation [41,42][41][42]. Moreover, SR-B1 in macrophages and endothelial cells could also promote the uptake by HDL of modified lipoproteins that contribute to the development of early atherosclerotic lesions [43,44,45][43][44][45]. Hepatic SR-B1 mediates the clearance of VLDL, LDL, and Lp(a), whose accumulation in plasma facilitate the progression of atherosclerosis [16,17,18,19][16][17][18][19]. SR-B1 promotes the reduction of apoptosis, and mediates efferocytosis of apoptotic cells in macrophages of atherosclerotic lesions [46,47][46][47]. Platelet SR-B1 has been implicated as a negative controller in the development of thrombosis [48,49][48][49] (Figure 1) (Table 1).
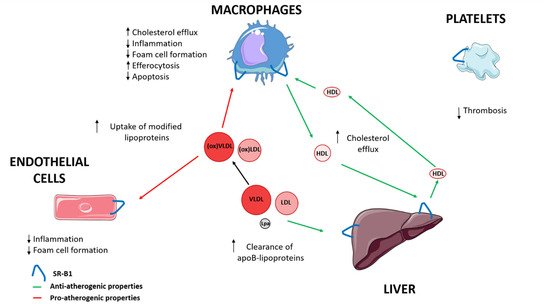
Figure 1. The role of SR-B1 in progression of atherosclerosis. LDL, low-density lipoprotein; Lp(a), lipoprotein (a); VLDL, very low-density lipoprotein; ↑ increase; ↓ decrease.
Table 1. The role of SR-B1 in atherosclerosis. LDL, low-density lipoprotein; Lp(a), lipoprotein (a); VLDL, very low-density lipoprotein; ↑ increase; ↓ decrease.
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics’2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603.
- Castelli, W.P.; Garrison, R.J.; Wilson, P.W.F.; Abbott, R.D.; Kalousdian, S.; Kannel, W.B. Incidence of Coronary Heart Disease and Lipoprotein Cholesterol Levels: The Framingham Study. JAMA J. Am. Med. Assoc. 1986, 256, 2835–2838.
- Hoekstra, M. SR-BI as target in atherosclerosis and cardiovascular disease—A comprehensive appraisal of the cellular functions of SR-BI in physiology and disease. Atherosclerosis 2017, 258, 153–161.
- Linton, M.F.; Tao, H.; Linton, E.F.; Yancey, P.G. SR-BI: A Multifunctional Receptor in Cholesterol Homeostasis and Atherosclerosis. Trends Endocrinol. Metab. 2017, 28, 461–472.
- Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; Packard, C.J.; et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA J. Am. Med. Assoc. 2009, 302, 1993–2000.
- Assmann, G.; Cullen, P.; Schulte, H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation 2002, 105, 310–315.
- Brunham, L.R.; Hayden, M.R. Human genetics of HDL: Insight into particle metabolism and function. Prog. Lipid Res. 2015, 58, 14–25.
- Helgadottir, A.; Sulem, P.; Thorgeirsson, G.; Gretarsdottir, S.; Thorleifsson, G.; Jensson, B.Ö.; Arnadottir, G.A.; Olafsson, I.; Eyjolfsson, G.I.; Sigurdardottir, O.; et al. Rare SCARB1 mutations associate with high-density lipoprotein cholesterol but not with coronary artery disease. Eur. Heart J. 2018, 39, 2172–2178.
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580.
- Holmes, M.V.; Ala-Korpela, M.; Smith, G.D. Mendelian randomization in cardiometabolic disease: Challenges in evaluating causality. Nat. Rev. Cardiol. 2017, 14, 577–599.
- Schwartz, G.G.; Olsson, A.G.; Abt, M.; Ballantyne, C.M.; Barter, P.J.; Brumm, J.; Chaitman, B.R.; Holme, I.M.; Kallend, D.; Leiter, L.A.; et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 2012, 367, 2089–2099.
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.P.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.C.; Waters, D.D.; et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007, 357, 2109–2122.
- Van der Steeg, W.A.; Holme, I.; Boekholdt, S.M.; Larsen, M.L.; Lindahl, C.; Stroes, E.S.G.; Tikkanen, M.J.; Wareham, N.J.; Faergeman, O.; Olsson, A.G.; et al. High-Density Lipoprotein Cholesterol, High-Density Lipoprotein Particle Size, and Apolipoprotein A-I: Significance for Cardiovascular Risk. The IDEAL and EPIC-Norfolk Studies. J. Am. Coll. Cardiol. 2008, 51, 634–642.
- Chroni, A.; Kardassis, D. HDL Dysfunction Caused by Mutations in apoA-I and Other Genes that are Critical for HDL Biogenesis and Remodeling. Curr. Med. Chem. 2018, 26, 1544–1575.
- Acton, S.; Rigotti, A.; Landschulz, K.T.; Xu, S.; Hobbs, H.H.; Kriegert, M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science (80-.) 1996, 271, 518–520.
- Acton, S.L.; Scherer, P.E.; Lodish, H.F.; Krieger, M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J. Biol. Chem. 1994, 269, 21003–21009.
- Calvo, D.; Gómez-Coronado, D.; Lasunción, M.A.; Vega, M.A. CLA-1 is an 85-kD plasma membrane glycoprotein that acts as a high- affinity receptor for both native (HDL, LDL, and VLDL) and modified (OxLDL and AcLDL) lipoproteins. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2341–2349.
- Yang, X.P.; Amar, M.J.; Vaisman, B.; Bocharov, A.V.; Vishnyakova, T.G.; Freeman, L.A.; Kurlander, R.J.; Patterson, A.P.; Becker, L.C.; Remaley, A.T. Scavenger receptor-BI is a receptor for lipoprotein(a). J. Lipid Res. 2013, 54, 2450–2457.
- Utermann, G. The mysteries of lipoprotein(a). Science (80-.) 1989, 246, 904–910.
- Al-Jarallah, A.; Trigatti, B.L. A role for the scavenger receptor, class B type I in high density lipoprotein dependent activation of cellular signaling pathways. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 1239–1248.
- Tarantino, G.; Citro, V.; Capone, D. Nonalcoholic Fatty Liver Disease: A Challenge from Mechanisms to Therapy. J. Clin. Med. 2019, 9, 15.
- Qiu, Y.; Liu, S.; Chen, H.T.; Yu, C.H.; Teng, X.D.; Yao, H.T.; Xu, G.Q. Upregulation of caveolin-1 and SR-B1 in mice with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 630–636.
- Krieger, M. Charting the fate of the “good cholesterol”: Identification and characterization of the high-density lipoprotein receptor SR-BI. Annu. Rev. Biochem. 1999, 68, 523–558.
- Connelly, M.A.; Williams, D.L. Scavenger receptor BI: A scavenger receptor with a mission to transport high density lipoprotein lipids. Curr. Opin. Lipidol. 2004, 15, 287–295.
- Rigotti, A.; Miettinen, H.E.; Krieger, M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr. Rev. 2003, 24, 357–387.
- Shen, W.-J.; Azhar, S.; Kraemer, F.B. SR-B1: A Unique Multifunctional Receptor for Cholesterol Influx and Efflux. Annu. Rev. Physiol. 2018, 80, 95–116.
- Shen, W.J.; Asthana, S.; Kraemer, F.B.; Azhar, S. Scavenger receptor B type 1: Expression, molecular regulation, and cholesterol transport function. J. Lipid Res. 2018, 59, 1114–1131.
- Shen, W.J.; Hu, J.; Hu, Z.; Kraemer, F.B.; Azhar, S. Scavenger Receptor class B type i (SR-BI): A versatile receptor with multiple functions and actions. Metabolism 2014, 63, 875–886.
- Gaidukov, L.; Nager, A.R.; Xu, S.; Penman, M.; Krieger, M. Glycine dimerization motif in the N-terminal transmembrane domain of the high density lipoprotein receptor SR-BI required for normal receptor oligomerization and lipid transport. J. Biol. Chem. 2011, 286, 18452–18464.
- Sahoo, D.; Darlington, Y.F.; Pop, D.; Williams, D.L.; Connelly, M.A. Scavenger receptor class B Type I (SR-BI) assembles into detergent-sensitive dimers and tetramers. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2007, 1771, 807–817.
- Marques, P.E.; Nyegaard, S.; Collins, R.F.; Troise, F.; Freeman, S.A.; Trimble, W.S.; Grinstein, S. Multimerization and Retention of the Scavenger Receptor SR-B1 in the Plasma Membrane. Dev. Cell 2019, 50, 283–295.
- Rodrigueza, W.V.; Thuahnai, S.T.; Temel, R.E.; Lund-Katz, S.; Phillips, M.C.; Williams, D.L. Mechanism of scavenger receptor class B type I-mediated selective uptake of cholesteryl esters from high density lipoprotein to adrenal cells. J. Biol. Chem. 1999, 274, 20344–20350.
- Neculai, D.; Schwake, M.; Ravichandran, M.; Zunke, F.; Collins, R.F.; Peters, J.; Neculai, M.; Plumb, J.; Loppnau, P.; Pizarro, J.C.; et al. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature 2013, 504, 172–176.
- Yancey, P.G.; De La Llera-Moya, M.; Swarnakar, S.; Monzo, P.; Klein, S.M.; Connelly, M.A.; Johnson, W.J.; Williams, D.L.; Rothblat, G.H. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J. Biol. Chem. 2000, 275, 36596–36604.
- Mineo, C. Lipoprotein Receptor Signaling in Atherosclerosis. Cardiovasc. Res. 2020, 116, 1254–1274.
- Reaven, E.; Chen, Y.D.I.; Spicher, M.; Azhar, S. Morphological evidence that high density lipoproteins are not internalized by steroid-producing cells during in situ organ perfusion. J. Clin. Investig. 1984, 74, 1384–1397.
- Oram, J.F.; Lawn, R.M.; Garvin, M.R.; Wade, D.P. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 2000, 275, 34508–34511.
- Sankaramarayanan, S.; Oram, J.F.; Asztalos, B.F.; Vaughan, A.M.; Lund-Katz, S.; Adorni, M.P.; Phillips, M.C.; Rothblat, G.H. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J. Lipid Res. 2009, 50, 275–284.
- Lim, H.Y.; Thiam, C.H.; Yeo, K.P.; Bisoendial, R.; Hii, C.S.; McGrath, K.C.Y.; Tan, K.W.; Heather, A.; Alexander, J.S.J.; Angeli, V. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-Mediated transport of HDL. Cell Metab. 2013, 17, 671–684.
- McGrath, K.C.Y.; Li, X.H.; Puranik, R.; Liong, E.C.; Tan, J.T.M.; Dy, V.M.; Dibartolo, B.A.; Barter, P.J.; Rye, K.A.; Heather, A.K. Role of 3β-hydroxysteroid-Δ24 reductase in mediating antiinflammatory effects of high-density lipoproteins in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 877–882.
- Vergeer, M.; Korporaal, S.J.A.; Franssen, R.; Meurs, I.; Out, R.; Hovingh, G.K.; Hoekstra, M.; Sierts, J.A.; Dallinga-Thie, G.M.; Motazacker, M.M.; et al. Genetic Variant of the Scavenger Receptor BI in Humans. N. Engl. J. Med. 2011, 364, 136–145.
- Yancey, P.G.; Jerome, W.G.; Yu, H.; Griffin, E.E.; Cox, B.E.; Babaev, V.R.; Fazio, S.; Linton, M.F. Severely altered cholesterol homeostasis in macrophages lacking apoE and SR-BI. J. Lipid Res. 2007, 48, 1140–1149.
- Van Eck, M.; Bos, I.S.T.; Hildebrand, R.B.; Van Rij, B.T.; Van Berkel, T.J.C. Dual role for scavenger receptor class B, type I on bone marrow-derived cells in atherosclerotic lesion development. Am. J. Pathol. 2004, 165, 785–794.
- Huang, L.; Chambliss, K.L.; Gao, X.; Yuhanna, I.S.; Behling-Kelly, E.; Bergaya, S.; Ahmed, M.; Michaely, P.; Luby-Phelps, K.; Darehshouri, A.; et al. SR-B1 drives endothelial cell LDL transcytosis via DOCK4 to promote atherosclerosis. Nature 2019, 569, 565–569.
- Yu, L.; Dai, Y.; Mineo, C. Novel Functions of Endothelial Scavenger Receptor Class B Type I. Curr. Atheroscler. Rep. 2021, 23.
- Tao, H.; Yancey, P.G.; Babaev, V.R.; Blakemore, J.L.; Zhang, Y.; Ding, L.; Fazio, S.; Linton, M.F. Macrophage SR-BI mediates efferocytosis via Src/PI3K/Rac1 signaling and reduces atherosclerotic lesion necrosis. J. Lipid Res. 2015, 56, 1449–1460.
- Mineo, C.; Shaul, P.W. Novel biological functions of high-density lipoprotein cholesterol. Circ. Res. 2012, 111, 1079–1090.
- Dole, V.S.; Matuskova, J.; Vasile, E.; Yesilaltay, A.; Bergmeier, W.; Bernimoulin, M.; Wagner, D.D.; Krieger, M. Thrombocytopenia and platelet abnormalities in high-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1111–1116.
- Korporaal, S.J.A.; Meurs, I.; Hauer, A.D.; Hildebrand, R.B.; Hoekstra, M.; Ten Cate, H.; Praticò, D.; Akkerman, J.W.N.; Van Berkel, T.J.C.; Kuiper, J.; et al. Deletion of the high-density lipoprotein receptor scavenger receptor BI in mice modulates thrombosis susceptibility and indirectly affects platelet function by elevation of plasma free cholesterol. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 34–42.
More
