Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dimitrios A. Lamprou and Version 1 by Edward Weaver.
Peptides possess multifunctional roles within therapeutic formulations, ranging from enhancing target specificity to acting as the active component of the medicine. By applying the platform of microfluidics, the range of applications for peptides has been seen to rise exponentially. Various therapeutic applications of peptides are listed in this review.
- microfluidics
- peptides
- proteins
- nanoparticles
- nanomedicines
- liposomes
1. Introduction
Peptide-based molecules fall into two distinct classes. The first class consist of peptides, which are short chains of amino acids (AAs), generally considered to be 2–50 AAs in length [1,2,3], that depending on their chemical composition, can perform a wide variety of functions, both for diagnostic and therapeutic purposes. With 22 well-known proteinogenic AAs [4] and many more nonproteinogenic AAs, the variability of peptide composition is extremely vast. Proteinogenic AAs are essential for normal human function, regulating metabolism, growth and repair, among other processes [5]; however, they can also be exploited for their medicinal qualities. Over 7000 naturally occurring peptides have been identified in nature [6], with much more able to be synthesized artificially, providing a huge library of molecules with potentially life-sustaining effects. Common peptide-based medicines, such as adalimumab (TNF inhibitor) and insulin, are used daily by millions of people for an extensive range of conditions, namely Crohn’s disease, diabetes mellitus, and rheumatoid arthritis, among others. The second class of peptide-based molecules consists of proteins, generally >50 AAs and can have complex secondary structures. This class includes enzymes and antibodies.
Peptide therapeutics are some of the most valuable medicines available [6]; however, their formulations are far from optimized. All peptide medicines must be delivered parenterally [7], often intravenously, due to the degradative process that occurs upon oral administration, which, despite being less convenient for the patient, still allows the delivery of life-saving medicines. Peptide drugs frequently possess high selectivity and potency while sustaining an agreeable safety profile, for example, novel antimicrobial peptides [8,9] and chemotherapeutic agents [10]. Their chemical nature often makes their metabolism predictable [11], which is very useful for administration, distribution, metabolism, and excretion (ADME) calculations; however, frequently, the unmodified peptide will have a very unfavorable ADME profile. For this reason, administrative formulation methods like nanoencapsulation, pegylation and peptide-implant reservoirs must be exploited to allow optimized delivery of the peptides.
2. Therapeutic Applications of Peptides
The application of peptides for medicinal use has exploded in recent years owing to their immense potential for therapeutic effect. Such applications include monoclonal antibodies (MABs) for cancer therapy and infectious disease prevention [30], vaccine synthesis using RNA (including the COVID-19 vaccine) [31,32] and newer uses for regenerative medicines [33], to name a few. A common method to circumvent peptide-based formulation issues is by formulating the peptides within a nanocarrier vessel, for instance, a nanoparticle (NP). Different NPs have been synthesized in an attempt to effectively manipulate peptide APIs, such as hybrid-magnetic NPs [34], nanoemulsions, liposomes, solid lipid nanoparticles (SLNs) [35] and polymer NPs (e.g., chitosan) [36]. All forms of NP have their own merits and drawbacks, and the method of synthesis can have a large impact on particle characteristics, irrespective of the type of NP being produced. The NP allows a certain level of customization to the medicine, offering the opportunity to provide target-specific delivery via either the NP’s shell material properties, or by the modification of the shell with specific chemical groups to increase target receptor affinity. The NP also provides protection for the peptide against the broad-spectrum of peptidases present within the human body by providing a physical barrier to the degradative enzymes [37]. Formulation of peptides into NPs has been achieved in various ways. However, the processes are far from being optimized and often fall short compared to the standards of industrial medicine synthesis that are implemented for other medicines. Methods, such as unilamellar fusion and thin-film hydration, have been proven to encapsulate peptides [38], but the methods have not yet rectified issues surrounding effective control of particle size and polydispersity index (PDI), as well as possessing unfavorable encapsulation efficiencies [39]. Thus, it has been theorized that a highly controllable system like Microfluidics (MFs) could be the solution to providing a high standard of encapsulation of peptide molecules within various NPs. A brief summary of method attributes is shown in Table 1.Table 1. Advantages and disadvantages of three methods used for peptide nanomedicine synthesis.
| Microfluidics | Unilamellar Vesicle Fusion | Thin-Film Hydration |
|---|---|---|
| Advantages | ||
| Allows synthesis at room temperature | Simple method | Simple method |
| Good control over PDI and particle size | Process can be controlled via electrostatic manipulation [40] | Acceptable encapsulation efficiencies for smaller peptides |
| Easy method to scale-up | ||
| High encapsulation efficiencies [41] | ||
| Continuous process | ||
| Disadvantages | ||
| Material interactions with MF chip | Less control over PDI and particle size | Less control over PDI and particle size |
| Requires initial high-cost system | Affected greatly by temperature | Produces heterogeneous particle population that requires extrusion/sonication [42] |
| Batch process | ||
2.1. Peptide Encapsulation
Formulation of peptides within liposomes is possible using MFs due to the ability of certain lipids, e.g., 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), to self-assemble into lipid nanostructures upon contact with aqueous media. Due to the amphiphilic nature of phospholipids, when in the presence of an aqueous medium, self-assembly occurs caused by noncovalent interactions forcing lipophilic areas of the molecules to a geometric state of low entropy [51], as seen in Figure 1. This principle has been harnessed during a novel attempt to encapsulate large biologic drugs, for example, ovalbumin and bovine serum albumin (BSA) [41]. The encapsulation of biologic drugs performed in this study produced liposomes of drastically smaller sizes and PDIs than obtained via previous methods, for instance, sonication or extrusion [41], as well as importantly increasing encapsulation efficacy.
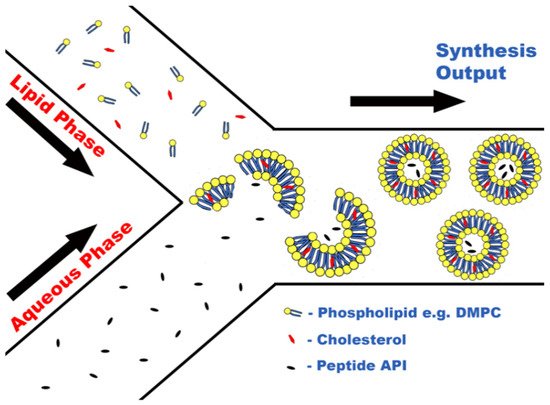

Figure 1. Concept of peptide encapsulation within liposomes via liposome self-assembly.
Increasing the encapsulation efficiency is crucial for biologic molecules due to their cost as even commonly used biologics, such as insulin, can cost over GBP 200 for 50 mg, which has been a limiting factor for scaling up production of previous methods to industrial levels. With further research encapsulating other biologics, it is possible that a financially viable method could be devised to produce these biologic formulations, which could even lead to groundbreaking medicines, including orally active insulin preparations. Evidently, as research in this area is limited, it still requires much work to determine the exact MF parameters that will be optimal for biologic encapsulation, or even that biologics are susceptible to encapsulation via an MF route. However, the evidence suggests so far that MFs offer a very promising synthetic method to produce such formulations.
2.2. Hydrogels and Peptide Antibiotics
The production of peptide-based biomaterials via MFs is attractive as they possess a low level of immunogenicity, and prolonged-release formulations, such as biologic hydrogels, will degrade slowly over time, via natural processes, into non-toxic materials [52,53]. Recent work performed utilizing the prolonged curative properties of peptide hydrogels allowed a double-pronged approach to wound healing via an MF-assisted process [54]. Peptide hydrogels possess a structure similar to the extracellular matrix, making them an ideal biocompatible medium for the controlled release of APIs. MFs were employed to synthesize a compact fibrous network of alginate to provide an optimal setting for the self-assembly of peptides onto the structure. The use of peptides on the structure also allowed the addition of a secondary API, the antibiotic lincomycin, within the hydrogel; hence providing two APIs to provide a therapeutic effect. The novel approach of using peptides within the structure ensured that the wound healing process could be optimized by providing both the anti-inflammatory benefits of prolonged-release peptide therapy, as well as introducing antibiotic activity.
It is possible that the process designed by Jain et al. [55] would be adaptable to a microfluidic approach, which exploited ultrashort peptides (USPs) to control gold NP synthesis within a complex hydrogel structure. USPs are short chains of amino acids (approximately 3–7 amino acids) that can be used to provide unique interactions with external environments. Owing to the predictable chemical behavior of peptides, they have been shown to be exploited to assist with NP shape control. NP shape is an important factor to consider as it affects overall PDI values, and it can also affect the thermal stability of a formulation or encapsulation efficacy. For example, nickel-based NPs in a spherical shape have better heat-transference properties than a disc shape [56].
Owing to increased pathogen drug resistance, the production of novel antimicrobials is limited, which is why a new area of antimicrobials in the form of peptide therapeutics should be explored. Despite the categorization of over 3000 AMPs, currently, only 7 antimicrobial peptides (AMPs) are approved for clinical use by the FDA [57], owing mainly to a lack of clinical efficacy. Bioassays performed using an MF design allow detecting antimicrobial activity. However, when compared to the traditional format of measuring via 96-well plate analysis, the results obtained were dissimilar, owing to differing oxygenation levels during incubation subjected to each system. The 96-well plate was exposed to a greater level of oxygen than the MFs assays, causing incomparability of the systems and hence the results of this study were inconclusive [57]. However, there is strong potential for future research in this area to explore different approaches for obtaining AMP activity using MFs. Conceptually, it is clear that MFs can be employed for this purpose, and there is an acute need for a new bioassay method, so this is an area that should be developed further.
2.3. Gene Therapy
Gene therapy is an area of medicine that could potentially have a massive impact on our perceived view of gold-standard healthcare. However, it is constantly shrouded by controversy and negative implications, as well as proving to be a difficult procedure to perfect. The advancement of MF designs could impact this area as current investigations into the MF synthesis of double-stranded DNA (dsDNA) carriers are at the forefront of enhancing gene delivery to specific targets [58]. Carrier materials like graphene oxide (GO) are versatile materials that, when unmodified, possess the ability to integrate single-stranded DNA or RNA onto their structure via distributed π−π
interactions. Modification via MFs is required to alter the charge of the GO structure to allow dsDNA bonding, as seen in Figure 2. Coating of the GO with a cationic lipid, such as 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), via MFs, increases the overall surface electrostatic charge, complementary to that of dsDNA. This lipid coating increased cellular uptake of DNA from 48.8% ± 6 using unmodified GO to an improved 93.9% ± 0.2. As one of the main inhibitors of gene therapy is achieving suitable gene concentrations for therapeutic effect, this progress is revolutionary. The carrier is required to prevent the susceptible DNA from degradation upon administration, as well as increasing gene transfection [59].
2.4. Oral Administration of Peptide-Based Medicines
Finally, for peptide therapeutics, a zealous approach to the synthesis of orally administered peptide medicines was performed using a multilamellar delivery system for the co-administration of an anti-diabetic peptide, glucagon-like peptide-1 (GLP-1), alongside another molecule, dipeptidyl peptidase 4 inhibitors (DPP4i) [60]. Currently, the only administration route for GLP-1 is parenteral, which leads to lower patient medicine adherence [61]. The natural degradation time for GLP-1 post-administration is approximately 2 min [62] due to rapid degradation by the enzyme dipeptidyl peptidase 4. Upon formulation into the dual-action functionalized NPs, hypoglycemic effects of the GLP-1 could still be observed 6 h after administration.
The synthesis of this formulation is complex, requiring multiple stages and multiple vessel materials: An initial NP was fabricated from PLGA and mesoporous silicon (PSi). This NP had additional surface functional groups of chitosan (CS) (to aid mucoadhesion) and cell-penetrating peptides (CPP), which assist the delivery of NP contents through the lipid bilayer of a cell. This NP contained the API GLP-1 and can be seen in Figure 2. MFs were then employed to further encapsulate this NP within an enteric coating material, hydroxypropylmethylcellulose acetyl succinate (HPMC-AS). The DPPi4 was only added upon final encapsulation alongside the HPMC-AS, which protected the two APIs against the inhospitable conditions exhibited upon oral administration. As research into orally administered peptide-based medicines is developed, a wide range of new patient-friendly, peptide-based medicines could begin to emerge. According to a 2019 study, parenterally administered medicines consistently had greater non-adherence rates than oral formulations [61], potentially leading to a lower quality of life.
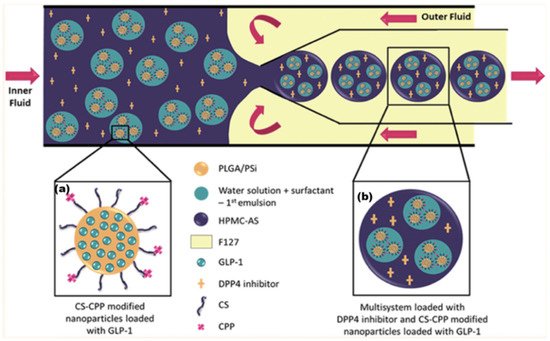

Figure 2. (a) Initial NP produced via the double emulsion method. (b) Final multilayered formulation produced by mixing emulsified particle (a) with HPMC-AS/DPP4i solution. Adapted with permission from Araújo et al. [60] Copyright 2015 American Chemical Society.
3. Conclusions
Microfluidics can used for drug discovery and development, and the market includes drug discovery, drug delivery, and in-vitro diagnostics, among many others. Microfluidics have also all the potentials to be used in the fight against a pandemic for the manufacturing of vaccines or rapid diagnosis. The microfluidic facility at the School of Pharmacy, hosts five microfluidic devices, including temperature controllers and high-speed microscope for the evaluation of the particles and flows in the microfluidic chips, including the manufacturing of chips by using 3D printing technologies.
