Homologous recombination (HR) is a fundamental evolutionarily conserved process that plays prime role(s) in genome stability maintenance through DNA repair and through the protection and resumption of arrested replication forks. HR promotes the exchange between homologous DNA sequences resulting in a novel combination of the genetic material. Therefore, HR is essential in genome stability maintenance but also plays an important role in genome diversity; such as in the case of meiosis. Many HR genes are deregulated in cancer cells. Notably, the breast cancer genes BRCA1 and BRCA2, two important HR players, are the most frequently mutated genes in familial breast and ovarian cancer.
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Homologous recombination (HR), promotes the exchange between homologous DNA sequences resulting in a novel combination of the genetic material, is a molecular process highly conserved through evolution that plays prominent roles in genome plasticity. Indeed, the DNA repair function(s) and the outcomes of HR are essential not only to maintain genome stability but also to promote genome variability [1][2][3][4][5]. HR allows the repair of different types of DNA lesions, mainly DNA double-strand breaks (DSBs) and interstrand crosslinks (ICLs) [6][7]. Moreover, an important role of HR in genome stability maintenance is the protection and resumption of arrested replication forks. However, prolonged replication fork arrest leads to DSBs that can thus be processed by HR [8][9].
Homologous recombination (HR), promotes the exchange between homologous DNA sequences resulting in a novel combination of the genetic material, is a molecular process highly conserved through evolution that plays prominent roles in genome plasticity. Indeed, the DNA repair function(s) and the outcomes of HR are essential not only to maintain genome stability but also to promote genome variability [1,2,3,4,5]. HR allows the repair of different types of DNA lesions, mainly DNA double-strand breaks (DSBs) and interstrand crosslinks (ICLs) [6,7]. Moreover, an important role of HR in genome stability maintenance is the protection and resumption of arrested replication forks. However, prolonged replication fork arrest leads to DSBs that can thus be processed by HR [8,9].
DSBs are generally considered the most toxic lesion. DSBs can be generated by endogenous stresses resulting from cellular metabolism, such as replication stress and reactive oxygen species (ROS), as well as from exogenous factors, such as ionizing radiation and chemotherapy agents (e.g., topoisomerase inhibitors). DSBs can also be programmed to trigger beneficial genomic rearrangements during meiotic differentiation [10] or the establishment of the immune system [11]. HR is also a driving force for the evolution of multigene families [12]. Therefore, thanks to this versatility, HR is involved in many fundamental biological processes. Finally, the technical application of HR constitutes the basis of targeted gene replacement for gene therapy as well as for the precise design of engineered organisms [13][14].
DSBs are generally considered the most toxic lesion. DSBs can be generated by endogenous stresses resulting from cellular metabolism, such as replication stress and reactive oxygen species (ROS), as well as from exogenous factors, such as ionizing radiation and chemotherapy agents (e.g., topoisomerase inhibitors). DSBs can also be programmed to trigger beneficial genomic rearrangements during meiotic differentiation [10] or the establishment of the immune system [11]. HR is also a driving force for the evolution of multigene families [12]. Therefore, thanks to this versatility, HR is involved in many fundamental biological processes. Finally, the technical application of HR constitutes the basis of targeted gene replacement for gene therapy as well as for the precise design of engineered organisms [13,14].
Genetic instability is a hallmark of aging and cancer [15][16][17]. Remarkably, markers of the DNA damage response have been found to be activated at pre-/early steps of tumorigenesis [18][19]. Since replication stress is a prominent endogenous source of DNA damage and genome instability, these data indicate a causal role of DNA replication stress in the early steps of tumor initiation [18][19][20]. Note that genetic instability can also fuel tumor progression. Because of its essential roles in genome stability maintenance, particularly in response to replication stress, HR is generally considered a tumor suppressor mechanism. Indeed, germline and somatic inactivating mutations in major HR actors have been observed in different types of tumors [21]. For example, germline heterozygous mutations in genes directly implicated in HR—
Genetic instability is a hallmark of aging and cancer [15,16,17]. Remarkably, markers of the DNA damage response have been found to be activated at pre-/early steps of tumorigenesis [18,19]. Since replication stress is a prominent endogenous source of DNA damage and genome instability, these data indicate a causal role of DNA replication stress in the early steps of tumor initiation [18,19,20]. Note that genetic instability can also fuel tumor progression. Because of its essential roles in genome stability maintenance, particularly in response to replication stress, HR is generally considered a tumor suppressor mechanism. Indeed, germline and somatic inactivating mutations in major HR actors have been observed in different types of tumors [21]. For example, germline heterozygous mutations in genes directly implicated in HR— BRCA1, BRCA2
,
PALB2, RAD51C
and
RAD51D—increase the risk of ovarian and breast cancer [22][23][24][25]. In addition, Fanconi anemia patients with biallelic mutations in
—increase the risk of ovarian and breast cancer [22,23,24,25]. In addition, Fanconi anemia patients with biallelic mutations in BRCA1, BRCA2
and
PALB2 show an increased risk of hematological malignancies and solid tumors [26][27][28][29][30][31][32][33].
show an increased risk of hematological malignancies and solid tumors [26,27,28,29,30,31,32,33].
2. Molecular Mechanisms of HR
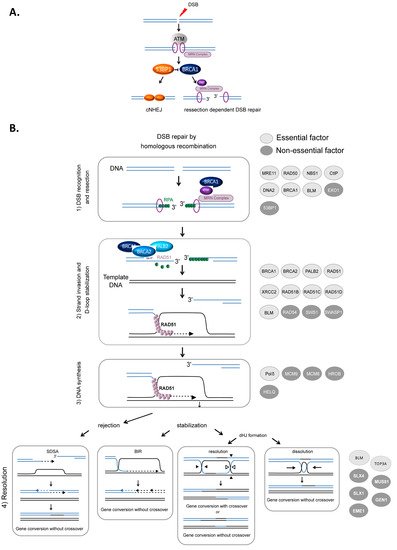
HR-mediated DSB repair represents a paradigm for the molecular steps of HR. DSBs are first recognized and signaled by the MRN complex (MRE11/RAD50/NBS1) in cooperation with the ATM (ataxia-telangiectasia mutated) kinase and the chromatin remodeling machinery. Subsequently, DSBs can be either repaired by canonical nonhomologous end-joining (cNHEJ) or resected (A) to then be repaired by HR or other resection-dependent DSB repair processes, such as single strand annealing (SSA) and alternative end-joining [34][35][36]. This choice between NHEJ and resection is controlled by the antagonistic activities of BRCA1, which favors resection, in competition with the protein complex 53BP1-Shieldin (53BP1-REV7-SHLD1-SHLD2-SHLD3), which prevents DNA end resection [37][38][39] (
A) to then be repaired by HR or other resection-dependent DSB repair processes, such as single strand annealing (SSA) and alternative end-joining [34,35,36]. This choice between NHEJ and resection is controlled by the antagonistic activities of BRCA1, which favors resection, in competition with the protein complex 53BP1-Shieldin (53BP1-REV7-SHLD1-SHLD2-SHLD3), which prevents DNA end resection [37,38,39] (A). The steps of HR that succeed DSB recognition can be summarized as follows (B): (1) DSB resection to generate a 3′ ssDNA stretch (see A); (2) RAD51 loading on the ssDNA, forming the ssDNA-RAD51 filament that promotes a search for homology and invasion of a homologous duplex DNA; (3) DNA synthesis primed by the invading 3′ end; and (4) the formation and resolution of HR intermediates that give rise to gene conversions (nonreciprocal exchange of genetic information) either associated with crossover or not (reciprocal exchange of adjacent sequences) (B) (reviewed in [1][3][5]).
B) (reviewed in [1,3,5]).
Figure 1.
HR-mediated DSB repair. (
A
). Signaling and competition between cNHEJ and resection [34]. The involvement of 53BP1, BRCA1 and the MRN complex. ( B
). Model of DSB repair by HR. (1) The MRN complex initiates a 5′ to 3′ resection under the control of BRCA1/CtIP, generating a 3′ single-stranded DNA (ssDNA) (
A
). (2) RAD51 is then loaded to the 3′ single-stranded DNA by the combined action of BRCA2/PALB2 and BRCA1, resulting in the formation of an ordered RAD51-ssDNA nucleofilament. The invasion of homologous duplex DNA by the RAD51-ssDNA filament induces the formation of a displacement loop (D-loop). (3) The invading strand primes DNA synthesis. (4) The D-loop can be either dismantled, leading to DSB repair by synthesis-dependent strand annealing (SDSA) or stabilized, leading to either DSB repair by BIR or the formation of a double Holliday junction (dHJ). The dHJ can be resolved or dissolved, leading to a repair product either associated with a crossover event or not. The proteins involved in each corresponding step are listed on the right side of the figure. Light gray circles indicate essential factors; essential factors are defined as genes whose knockout leads to embryonic lethality in mice. Dark gray circles indicate nonessential factors; these are genes whose KO does not lead to embryonic lethality in mice.
2.1. Resection
Resection, which is required to initiate HR, is performed in two steps (A). BRCA1 is recruited to DSB sites via its interaction with NBS1 and associates with BARD1, forming an active E3 ubiquitin ligase. This complex ubiquitinates the endonuclease CtIP, which cooperates with MRE11 to initiate DSB resection [40][41]. Then, exonuclease 1 (EXO1) and/or the BLM/DNA2 (helicase/nuclease) complex extend the 3′ overhang [42][43][44][45] (
A). BRCA1 is recruited to DSB sites via its interaction with NBS1 and associates with BARD1, forming an active E3 ubiquitin ligase. This complex ubiquitinates the endonuclease CtIP, which cooperates with MRE11 to initiate DSB resection [40,41]. Then, exonuclease 1 (EXO1) and/or the BLM/DNA2 (helicase/nuclease) complex extend the 3′ overhang [42,43,44,45] (B). Finally, the 3′ ssDNA stretch created by resection is coated with replication protein A (RPA), protecting it (B).
2.2. Loading RAD51 on ssDNA, Search for Homology and Strand Invasion
The loading of RAD51 onto ssDNA is performed by the BRCA2-PALB2 complex [46][47]. This protein complex interacts with BRCA1 and catalyzes the replacement of RPA by RAD51 on the stretch of 3′ ssDNA, creating the RAD51-ssDNA presynaptic complex [48][49][50]. Note that BRCA1 plays roles during different steps of HR: the initiation of resection and the loading of RAD51 (
The loading of RAD51 onto ssDNA is performed by the BRCA2-PALB2 complex [46,47]. This protein complex interacts with BRCA1 and catalyzes the replacement of RPA by RAD51 on the stretch of 3′ ssDNA, creating the RAD51-ssDNA presynaptic complex [48,49,50]. Note that BRCA1 plays roles during different steps of HR: the initiation of resection and the loading of RAD51 (B) [3][51][52].
B) [3,51,52].
The ssDNA-RAD51 filament scans the genome to search for homology. Once a homologous sequence is found, the filament invades the duplex homologous DNA and initiates strand exchange, creating a displacement loop (D-loop).
2.3. DNA Synthesis
The 3′ invading strand primes DNA synthesis through the recruitment of DNA and the copy of the invaded DNA molecule. Numerous studies have demonstrated the involvement of many polymerases in this process, although Polδ has been proposed to play a primary role [53]. The protein complexes HROB-MCM8–MCM9 and HELQ are proposed to have redundant helicase functions to promote DNA synthesis during HR [54].
2.4. Formation and Resolution of HR Intermediates
Strand invasion and DNA synthesis lead to the formation of different intermediates whose processing leads to gene conversion either associated with crossover products or not (). The invading strand can be disassembled, channeling DSB repair toward synthesis-dependent strand annealing (SDSA) (B). If stabilized, the D-loop can lead to DSB repair by break-induced repair (BIR) or to the formation of double Holliday junctions that can be either dissolved by the BLM-TOP3A-RMI1/2 complex or resolved by the structure-specific resolvases MUS81-EME1, GEN1 or SLX1 (B) (reviewed in [2][55]).
B) (reviewed in [2,55]).
Of note, the DNA helicase BLM, which is mutated in Bloom syndrome, plays several roles, sometimes contradictory, and at different HR steps. Indeed, BLM is involved in different steps of HR, including end resection at HR initiation [42][56], D-loop rejection and dHJ resolution at HR termination [56][57]. At resection initiation, depending on the cell cycle phase that modifies its interacting partners, BLM either favors the loading of 53BP1 on the DSB in G1 phase, preventing the initiation of unscheduled resection, or, in contrast, favors resection in S phase when interacting with TOP3 [58].
Of note, the DNA helicase BLM, which is mutated in Bloom syndrome, plays several roles, sometimes contradictory, and at different HR steps. Indeed, BLM is involved in different steps of HR, including end resection at HR initiation [42,56], D-loop rejection and dHJ resolution at HR termination [56,57]. At resection initiation, depending on the cell cycle phase that modifies its interacting partners, BLM either favors the loading of 53BP1 on the DSB in G1 phase, preventing the initiation of unscheduled resection, or, in contrast, favors resection in S phase when interacting with TOP3 [58].
2.5. Accessory Proteins
RAD54, a member of the SWI2/SNF2 protein family (ATP-dependent chromatin remodelers), interacts with RAD51, and in vitro studies have proposed that it functions as a RAD51 cofactor [59][60][61]. RAD54 catalyzes the extension of joint molecules [62] and stabilizes the D-loop [63].
RAD54, a member of the SWI2/SNF2 protein family (ATP-dependent chromatin remodelers), interacts with RAD51, and in vitro studies have proposed that it functions as a RAD51 cofactor [59,60,61]. RAD54 catalyzes the extension of joint molecules [62] and stabilizes the D-loop [63].
A family of six proteins (RAD51B, RAD51C, RAD51D, XRCC2, XRCC3, and RAD51AP1), known as the RAD51 paralogs (i.e., proteins that share sequence homology with RAD51 in a given species), has been identified in mammals. Two distinct complexes have been identified: RAD51B–RAD51C–RAD51D–XRCC2 (BCDX2) and RAD51C–XRCC3 (CX3) [64]. RAD51 paralogs favor the recruitment of RAD51 to DNA damage sites [65] and promote the formation and stabilization of the RAD51 nucleoprotein filament. However, the exact role of each paralog remains to be fully determined. In addition, RAD51 paralogs influence gene conversion tract length [66][67]. The SWSAP1 protein, a noncanonical paralog of RAD51, forms the so-called SHU complex when associated with SWS1 (SWSAP1-SWS1). SHU interacts with RAD51 and regulates its function [68].
A family of six proteins (RAD51B, RAD51C, RAD51D, XRCC2, XRCC3, and RAD51AP1), known as the RAD51 paralogs (i.e., proteins that share sequence homology with RAD51 in a given species), has been identified in mammals. Two distinct complexes have been identified: RAD51B–RAD51C–RAD51D–XRCC2 (BCDX2) and RAD51C–XRCC3 (CX3) [64]. RAD51 paralogs favor the recruitment of RAD51 to DNA damage sites [65] and promote the formation and stabilization of the RAD51 nucleoprotein filament. However, the exact role of each paralog remains to be fully determined. In addition, RAD51 paralogs influence gene conversion tract length [66,67]. The SWSAP1 protein, a noncanonical paralog of RAD51, forms the so-called SHU complex when associated with SWS1 (SWSAP1-SWS1). SHU interacts with RAD51 and regulates its function [68].