Transthyretin (TTR) is a plasma homotetrameric protein that transports thyroxine and retinol. TTR itself, under pathological conditions, dissociates into partially unfolded monomers that aggregate and form fibrils. Metal ions such as Zn2+, Cu2+, Fe2+, Mn2+ and Ca2+ play a controversial role in the TTR amyloidogenic pathway. TTR is also present in cerebrospinal fluid (CSF), where it behaves as one of the major Aβ-binding-proteins. The interaction between TTR and Aβ is stronger in the presence of high concentrations of Cu2+. Crystals of TTR, soaked in solutions of physiological metals such as Cu2+ and Fe2+, but not Mn2+, Zn2+, Fe3+, Al3+, Ni2+, revealed an unusual conformational change.
- transthyretin
- neuroprotection
- metal ions
- altered conformations
1. The TTR structure
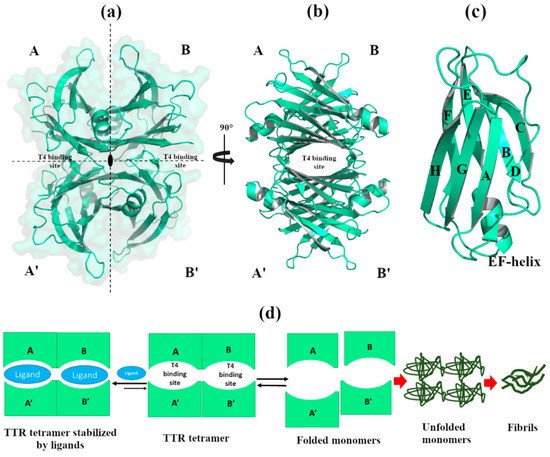
Figure 1.
a
b
c
d
2. Effect of Metals in TTR Structure and Function
2.1. Non-Physiological Metals: Cr
3+
and Re
2+
Starting from a previous study where two halides, iodine and chloride, were investigated for their ability to increase the stability of the TTR tetramer [5], T. Sato et al. screened several metal ions (Cu
2+
2+
2+
2+
2+
2+
3+
3+
3+ showed a significant reduction in amyloid fibril formation, favoring T4 binding with the tetramer, and stabilizing both wt-TTR and the V30M-TTR variant [6]. The stability of the tetramer was confirmed by calorimetric analyses performed at physiological and acid pH (25 µM TTR samples against 500 µM Cr
3+
3+ was solved at 1.8 Å, and the anomalous difference Fourier maps displayed major peaks close to Glu54 (data not deposited) [6]. The authors suggested that Cr
3+ can electrostatically neutralize this zone, pushing the T4 to bind TTR [6].
Crystal structures of wt-TTR, in complex with rhenium, were obtained by soaking TTR crystals in cryoprotectant solutions rich in tris-carbonyl derivatives, following a strategy already reported in the literature [7][8]. The initial purpose of this investigation was to study and try to solve the phase problem during the single-wavelength anomalous diffraction (SAD) experiments. Briefly, crystals were obtained by sitting-drop vapor-diffusion method from a reservoir solution of 21% polyethylene glycol 4000 (PEG4K), 0.14 M imidazole malate, pH 6.0 (PDB id:5K1J) as well as from 21% PEG4K, 0.14 M imidazole malate, pH 6.0, 3.6% polyethylene glycol monomethylether (MPEG5K), and 30 mM sodium acetate, pH 5.5 (PDB id 5K1N). For data collection, the first crystal was cryoprotected by soaking for 10 min into cryoprotectant solution composed of 40% of SM2 (12.5% ethylene glycol, 12.5% glycerol, 12.5% 1,2-propanediol, 25% DMSO and 37.5% 1,4-dioxane), 25% PEG 8K and 0.2 mM of rhenium derivative (PDB id:5K1J). The second one was cryoprotected with a solution of 40% SM3 (25% diethylene glycol, 25% ethylene glycol, 25% glycerol, 25% 1,4-dioxane), 25% PEG 8K and 0.5 mM of rhenium compound [7] (PDB id 5K1N). Interestingly, crystals soaked with a low concentration of rhenium derivatives gave structures that diffracted to 1.7 Å resolution, showing a new wt-TTR conformation. Both structures show a Re atom that coordinates with His88 in monomer B and B’,
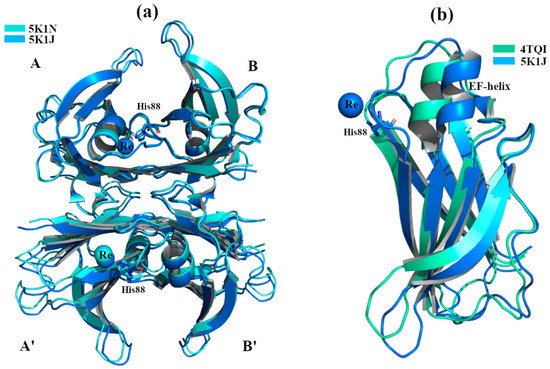
Figure 2.
2+
a
2+
2+
b
Figure 2a,b. This TTR conformation has never been observed before, even if its effect in the central channel is attributable to the well-known negative cooperativity of TTR [9].
1 polymorph (r.m.s.d. 2.74 Å) [7][10]. For more detailed information regarding the structural differences between the TTR-Re crystal complexes and other structures, we refer the reader to the original manuscript [7]. It has been hypothesized that this conformation obtained in the presence of Re, where the EF-helix swings away from the T4 binding site opening the dimer B-B’, may represent the tetramer conformation that is able to interact with the Aβ peptide. This consideration is strengthened by previous studies, where it has been observed that Leu82 (EF-helix) and Leu110 (strand G) are key residues for the interaction between TTR and Aβ [11][12].
2.2. Physiological Metals
2.2.1. Zn
2+
2+ binds to TTR, both in vitro and in vivo [13][14][15]. High levels of Zn
2+
2+
2+ was found [14]. Starting from this evidence, it was hypothesized that Zn
2+
X-ray crystal structure analyses of four engineered monomer TTRs (M-TTR, F87M/L110M) [16], in complex with Zn
2+
2+ binding sites (ZBS) [17]. Crystals were grown by hanging-drop vapour-diffusion method in a solution composed of 100 mM sodium citrate, 2.0 ammonium sulphate and 200 mM of zinc acetate. The C-α r.m.s.d. among the structures (PDB id: 3DGD, 3GPS, 3GRB, 3GRG) is less than 0.4 Å, indicating that they are very similar to each other,
2+
2+
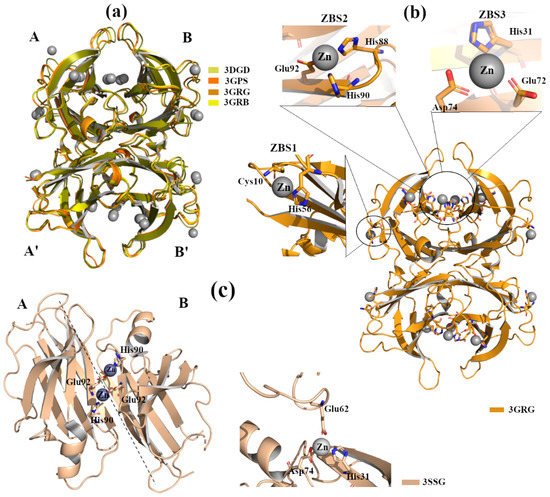
Figure 3.
2+
a
2+
b
c
Moreover, the occupation of ZBS1 does not produce any effect on TTR-RBP interaction (the residues of TTR involved in the binding with RBP are Arg21, Val20, Leu82 and Ile-84) [18]. In contrast, in the presence of a higher Zn
2+
2+
Figure 3b induces slight structural rearrangements around the α-helix at all tested pH. These modifications are comparable with those detected in other TTR structures crystallized without metals but at acidic pH [19]. Contrary to the effect observed for ZBS1, the involvement of ZBS2 and ZBS3 and their corresponding conformational changes affect the interaction of TTR with RBP [17].
TTR L55P is considered as one of the most aggressive amyloidogenic variants that accelerate pathology onset. Structural studies report that apo-TTR L55P, as well as TTR L55P in complex with 2,4-dinitrolphenol (DNP), possess the typical tetrameric structure. The only detected change is local in the monomers, where, due to a disorder of the short edge strand D, an extended loop between strands C and E appears [20][21].
2+
2+ [22]. TTR L55P, in complex with Zn
2+
2+
2+
2+
2
1
Recently, studies report that TTR can also be considered as an inducible metallopeptidase [15][23]. The TTR catalytic triad is composed of residues His88, His90 and Glu92, and its activation is modulated by bivalent metal ions. It has been demonstrated that, when TTR proteolytic activity is inhibited in vitro by metal chelators, Zn
2+
2+
2+
2+ only partially reactivated the enzyme. This TTR proteolytic activity agrees with the hypotheses in which TTR behaves as a protease in neurodegenerative diseases such as AD and atherosclerosis. In fact, apoA-I and Aß can be cleaved by TTR, which might affect the onset of atherosclerosis and AD, respectively [24][25].
2+
2.2.2. Cu2+, Fe2+ and Mn2+
2+ binds to TTR; the presence of the metal induces some structural and functional effects on TTR [13]. Binding of Cu
2+
2+ binds TTR in the presence of 1-anilino-8-naphthalene sulfonate (ANS). ANS is a small fluorescent ligand able to bind both T4 binding sites and stabilize the TTR tetrameric structure [26][27]. The observation of a fluorescence perturbation upon Cu
2+ binding suggests a local structural variation across the central channel [13]. Interestingly, the same study demonstrated that Cu
2+
2+
2+ favors tetramer dissociation and accelerates the process of amyloid formation [13].
As mentioned in the introduction, in contrast with its intrinsic amyloidogenic potential, TTR has a neuroprotective role in AD. TTR binds Aβ, participating in its clearance from the brain [28][29]. It has been hypothesized that metals might play a role in triggering this additional function of TTR.
2+
2+
2+
2
2
2
2+
2+
Figure 4b [30]. In contrast, when wt-TTR crystals were treated with Cu
2+
2+
2+ [7][30]. The electron density map suggests that Cu
2+
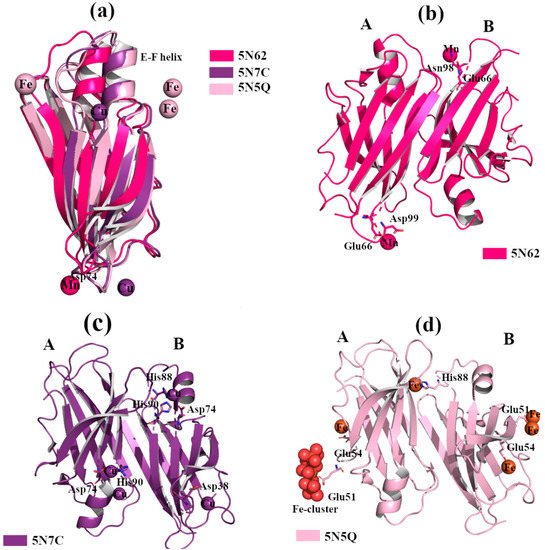
Figure 4.
2+
2+
2+
a
2+
2+
2+
2+
b
2+
c
2+
d
2+
2+
2+
2+
2+ crystal complex [30]. In order to verify if trivalent metals ions also induce conformational changes, several wt-TTR crystals were soaked with Al
3+
3+
3+
2+ with TTR protein was confirmed via the strong anomalous signal registered in the phased anomalous difference Fourier map [31][32]. The highest peak is registered close to Glu51, a second site is located near Glu54 at the entrance of the T4 binding site, and another peak is detected around His88,
Figure 4d. As previously seen in the rhenium-TTR structure, the conformational change affects only the β-strands E and F, and the short α-helix connecting them located in monomers B and B’ [30].
2+
2+, modifies the dimer-dimer interface involving the TTR amino acid sequence, which is implicated in the interaction with Aβ peptides [11][33]. It is known that in the brains of AD patients, the concentration of metals (in particular Cu, Fe and Zn) is altered, and the amount of Cu
2+ in Aβ plaque can reach 400 µM [34]. A bio-layer interferometry (BLI) study in solution revealed that a binding affinity between TTR and Aβ1-28 peptide is in nanomolar range when in the presence of Cu
2+
2+
2+ might be associated with TTR’s ability to neutralize Aβ [30]. This experimental evidence suggests that the conformational change induced by Cu
2+
2+ is not a structural artefact due to the soaking technique, but is probably related to the neuroprotective role that TTR possesses in the brain. This new conformation of TTR has inspired the design of PROteolysis-Targeting Chimeras (PROTAC) compounds that can induce the “active TTR conformation” favoring both the stabilization of TTR tetramer and the Aβ scavenger [35].
2.2.3. Ca2+
2+
2+ is a key factor in the triggering of neurodegenerative processes [36]. TTR binds Ca
2+ [37], and X-ray studies do not show any relevant structural changes in the wt-TTR tetrameric structure. TTR-Ca
2+
2
2+ [38]) and 34–40% PEG 400, 400 mM CaCl
2
2+) [39]. The superposition of the two structures reveals that there is a common Ca
2+
2+
2+, and in these cases no relevant structural differences were detected [38][40][41].
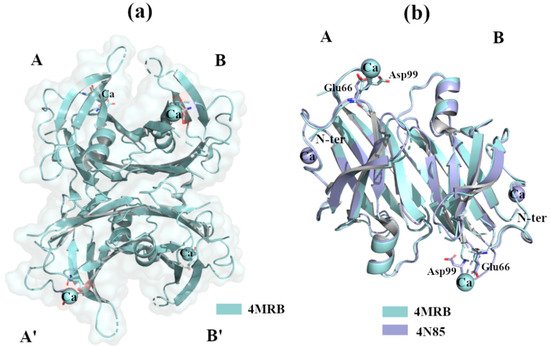
Figure 5.
2+
a
2+
b
2+
2+ does not modify the environment around tryptophan residues, confirming that it does not induce global structural changes [42]. Interestingly, in the presence of Ca
2+, the binding between TTR and ANS decreases, suggesting that the T4 binding sites are less accessible. Deeper analysis confirmed that the fluorescent emission spectra, recorded at 275 nm, did not display any significant structural modifications [42]. However, the same study confirms that Ca
2+
2+
