Cytokines are key molecules within the tumor microenvironment (TME) that can be used as biomarkers to predict the magnitude of anti-tumor immune responses. During immune monitoring, it has been customary to predict outcomes based on the abundance of a single cytokine, in particular IFN-γ or TGF-β, as a readout of ongoing anti-cancer immunity. However, individual cytokines within the TME can exhibit dual opposing roles. For example, both IFN-γ and TGF-β have been associated with pro- and anti-tumor functions. Moreover, cytokines originating from different cellular sources influence the crosstalk between CD4+ and CD8+ T cells, while the array of cytokines expressed by T cells is also instrumental in defining the mechanisms of action and efficacy of treatments. Thus, it becomes increasingly clear that a reliable readout of ongoing immunity within the TME will have to include more than the measurement of a single cytokine.
- T cell
- cytokines
- tumor
1. Introduction
[1]
[2]
[1]
[1]
[1]
[3]
[4]
[7]
[5]
[6]
[8]
[8]
[10]
[11]
+
+
+
+
+
[14]
[12]
+
[15]
2. T Cell-Derived Cytokines as Biomarkers of Anti-Tumor Activity
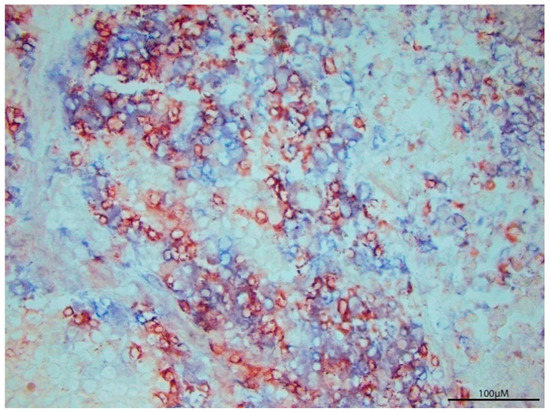
Figure 1.
[16]
[17]
[18]
[19]
[17]
[20]
[17]
[19]
[20]
+
[17]
[18]
[18]
[18]
[18]
[21]
[18]
[18]
[18]
[18]
[18]
[22]
[22]
[22]
[22]
[22]
[22]
+
+
[22]
[25]
[25]
[26]
[26]
+
[26]
[26]
[26]
[26]
[26]
Table 1.
| Study | Cytokines Probed and Monitoring Methods | Patients/Source of T Cells | Cancer Type and Treatment | T cell Treatment Outcome Response and Correlated Cytokine |
|---|---|---|---|---|
| #1 [27] | IFN-γ, IL-2, TNF-α FACS analysis, ICS, ELISpot |
Healthy donors | CD3+ T cells isolated from peripheral blood | IL-2 (mostly among CD8− T cells) and IFN-γ secreting cells increased, TNF-α secreting cells decreased. IFN-γ and IL-2 secreting cytokines showed functional state persistence. |
| #2 [28] | TNF-α IFN-γ, IL-10, IL-17, IL-2 Intracellular cytokine staining of CD4+ and CD8+ T cells, in renal parenchyma tissues |
Peripheral blood, fresh tumor, and autologous renal parenchyma | Renal cell carcinoma PBMC and TIL thawed and analyzed for cytokine release. |
IL-10 increased among CD4+ and CD8+ subsets; TNF-α (CD4+ and CD8+), IFN-γ (CD8+) increased after activation. Some patients had increased IL-17 in CD4+ TIL. CD107a surface expression found in CD8+ and some CD4+ cells post- activation. Cytokine secretion pattern of responders: TNF-α, IFN-γ, IL-2 with little IL-5. |
| #3 [29] | TNF, IFN-γ, CD107a (cytotoxicity marker). FACS analysis, cytotoxicity assays, phenotype analysis, flow cytometry |
Serial blood sample obtained from TIL treated patients | Melanoma IL-2 based TIL therapy |
CD8+ T cells expressing CD107a were fewer than cytokine producing cells. Most CD107a + cells also produced one cytokine. |
| #4 [30] | IFN-γ, TNF-α, CCL3 IFNγ ELISPOT assay, FlowJo |
22 CMV seropositive patients | Glioblastoma In vitro generation of (CMV) pp65 T cells and CMV pp65- DCs from PBMCs |
Patients receiving CMV pp65 T cells had more IFNγ+, TNFα+ CCL3+ pp65 specific CD8+ T cells. Survival in treated patients correlated with expression of IFNγ, TNFα and CCL3. |
| #5 [31]. | IFN-γ2,TNF-α1,2, IL-2, IL-12, IL-18, IL-21 CCL41,2, CD107a2(cytotoxicity marker) Flow cytometry, ELISA, Bio-Plex |
Bulk ascites cell preparations from high-grade serous EOC patients | Epithelial ovarian cancer (EOC) Exogenous cytokine therapy and introduction of EOC ascites environment on T—cell polyfunctionality |
IL-1+, IL-12+ IL-18 enhanced IFNγ (by CD8+ cells), TNF-α, and CCL4 expression Cytokine combination synergistically induced polyfunctional responses and decreased cytokine negative or monofunctional T cells. |
| #6 [32] | IFN-γ3, TNF-α3 IL-23 Flow cytometry, immunohistochemistry |
25 treatment- naïve NSCLC patients with clinical stage I-Iva tumors. | Non-small cell lung cancers (NSCLC) TIL therapy |
CD4+/CD8+ cells producing either 2 or 3 of the cytokines were most informative. TNFα and IL-2 were crucial to T cell mediated immunity. |
| Study | Cytokines Probed and Monitoring Methods | Patients/Source of T Cells | Cancer Type and Treatment | T cell Treatment Outcome Response and Correlated Cytokine |
|---|---|---|---|---|
| #1 [27] | IFN-γ, IL-2, TNF-α FACS analysis, ICS, ELISpot |
Healthy donors | CD3+ T cells isolated from peripheral blood | IL-2 (mostly among CD8− T cells) and IFN-γ secreting cells increased, TNF-α secreting cells decreased. IFN-γ and IL-2 secreting cytokines showed functional state persistence. |
| #2 [28] | TNF-α IFN-γ, IL-10, IL-17, IL-2 Intracellular cytokine staining of CD4+ and CD8+ T cells, in renal parenchyma tissues |
Peripheral blood, fresh tumor, and autologous renal parenchyma | Renal cell carcinoma PBMC and TIL thawed and analyzed for cytokine release. |
IL-10 increased among CD4+ and CD8+ subsets; TNF-α (CD4+ and CD8+), IFN-γ (CD8+) increased after activation. Some patients had increased IL-17 in CD4+ TIL. CD107a surface expression found in CD8+ and some CD4+ cells post- activation. Cytokine secretion pattern of responders: TNF-α, IFN-γ, IL-2 with little IL-5. |
| #3 [29] | TNF, IFN-γ, CD107a (cytotoxicity marker). FACS analysis, cytotoxicity assays, phenotype analysis, flow cytometry |
Serial blood sample obtained from TIL treated patients | Melanoma IL-2 based TIL therapy |
CD8+ T cells expressing CD107a were fewer than cytokine producing cells. Most CD107a + cells also produced one cytokine. |
| #4 [30] | IFN-γ, TNF-α, CCL3 IFNγ ELISPOT assay, FlowJo |
22 CMV seropositive patients | Glioblastoma In vitro generation of (CMV) pp65 T cells and CMV pp65- DCs from PBMCs |
Patients receiving CMV pp65 T cells had more IFNγ+, TNFα+ CCL3+ pp65 specific CD8+ T cells. Survival in treated patients correlated with expression of IFNγ, TNFα and CCL3. |
| #5 [31]. | IFN-γ2,TNF-α1,2, IL-2, IL-12, IL-18, IL-21 CCL41,2, CD107a2(cytotoxicity marker) Flow cytometry, ELISA, Bio-Plex |
Bulk ascites cell preparations from high-grade serous EOC patients | Epithelial ovarian cancer (EOC) Exogenous cytokine therapy and introduction of EOC ascites environment on T—cell polyfunctionality |
IL-1+, IL-12+ IL-18 enhanced IFNγ (by CD8+ cells), TNF-α, and CCL4 expression Cytokine combination synergistically induced polyfunctional responses and decreased cytokine negative or monofunctional T cells. |
| #6 [32] | IFN-γ3, TNF-α3 IL-23 Flow cytometry, immunohistochemistry |
25 treatment- naïve NSCLC patients with clinical stage I-Iva tumors. | Non-small cell lung cancers (NSCLC) TIL therapy |
CD4+/CD8+ cells producing either 2 or 3 of the cytokines were most informative. TNFα and IL-2 were crucial to T cell mediated immunity. |
References
- Foster, J.R. The functions of cytokines and their uses in toxicology. Int. J. Exp. Pathol. 2001, 82, 171–192.
- Bonini, C.; Mondino, A. Adoptive T-cell therapy for cancer: The era of engineered T cells. Eur. J. Immunol. 2015, 45, 2457–2469.
- Germano, G.; Allavena, P.; Mantovani, A. Cytokines as a key component of cancer-related inflammation. Cytokine 2008, 43, 374–379.
- Castro, F.; Cardoso, A.P.; Goncalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847.
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49.
- Jennifer Gillary Segal, N.C.L.; Tsung, Y.L.; Jeffrey, A.; Norton, K.T. The Role of IFN-y in Rejection of Established Tumors by IL-12: Source of Productiona and Target. Cancer Res. 2002, 62, 4696–4703.
- Le Poole, I.C.; Riker, A.I.; Quevedo, M.E.; Stennett, L.S.; Wang, E.; Marincola, F.M.; Kast, W.M.; Robinson, J.K.; Nickoloff, B.J. Interferon-gamma reduces melanosomal antigen expression and recognition of melanoma cells by cytotoxic T cells. Am. J. Pathol. 2002, 160, 521–528.
- Batlle, E.; Massague, J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940.
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the Treatment of Cancer. J. Interferon Cytokine Res. 2019, 39, 6–21.
- Lee, S.; Margolin, K. Cytokines in cancer immunotherapy. Cancers 2011, 3, 3856–3893.
- Anderson, K.G.; Stromnes, I.M.; Greenberg, P.D. Obstacles Posed by the Tumor Microenvironment to T cell Activity: A Case for Synergistic Therapies. Cancer Cell 2017, 31, 311–325.
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912.
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: Naive to memory and everything in between. Adv. Physiol. Educ. 2013, 37, 273–283.
- Yuen, G.J.; Demissie, E.; Pillai, S. B lymphocytes and cancer: A love-hate relationship. Trends Cancer 2016, 2, 747–757.
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596.
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17.
- Knutson, K.L.; Disis, M.L. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother. 2005, 54, 721–728.
- Vegran, F.; Apetoh, L.; Ghiringhelli, F. Th9 cells: A novel CD4 T-cell subset in the immune war against cancer. Cancer Res. 2015, 75, 475–479.
- Nishimura, T.; Iwakabe, K.; Sekimoto, M.; Ohmi, Y.; Yahata, T.; Nakui, M.; Sato, T.; Habu, S.; Tashiro, H.; Sato, M.; et al. Distinct Role of Antigen-specific T Helper Type 1 (Th1) and Th2 Cells in Tumor Eradication in Vivo. Rockefeller Univ. Press 1999, 190, 617–627.
- van Belzen, I.; Kesmir, C. Immune biomarkers for predicting response to adoptive cell transfer as cancer treatment. Immunogenetics 2019, 71, 71–86.
- Goswami, R.; Kaplan, M.H. A brief history of IL-9. J. Immunol. 2011, 186, 3283–3288.
- Guery, L.; Hugues, S. Th17 Cell Plasticity and Functions in Cancer Immunity. Biomed. Res. Int 2015, 2015, 314620.
- Badri, K.R.; Gao, L.; Hyjek, E.; Schuger, N.; Schuger, L.; Qin, W.; Chekaluk, Y.; Kwiatkowski, D.J.; Zhe, X. Exonic mutations of TSC2/TSC1 are common but not seen in all sporadic pulmonary lymphangioleiomyomatosis. Am. J. Respir. Crit. Care Med. 2013, 187, 663–665.
- Silva-Santos, B.; Serre, K.; Norell, H. γδ T cells in cancer. Nat. Rev. Immunol. 2015, 15, 683–691.
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22.
- Xu, H.-M. Th1 cytokine-based immunotherapy for cancer. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 482–494.
- Han, Q.; Bagheri, N.; Bradshaw, E.M.; Hafler, D.A.; Lauffenburger, D.A.; Love, J.C. Polyfunctional responses by human T cells result from sequential release of cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 1607–1612.
- Attig, S.; Hennenlotter, J.; Pawelec, G.; Klein, G.; Koch, S.D.; Pircher, H.; Feyerabend, S.; Wernet, D.; Stenzl, A.; Rammensee, H.G.; et al. Simultaneous infiltration of polyfunctional effector and suppressor T cells into renal cell carcinomas. Cancer Res. 2009, 69, 8412–8419.
- Donia, M.; Kjeldsen, J.W.; Andersen, R.; Westergaard, M.C.W.; Bianchi, V.; Legut, M.; Attaf, M.; Szomolay, B.; Ott, S.; Dolton, G.; et al. PD-1(+) Polyfunctional T Cells Dominate the Periphery after Tumor-Infiltrating Lymphocyte Therapy for Cancer. Clin. Cancer Res. 2017, 23, 5779–5788.
- Reap, E.A.; Suryadevara, C.M.; Batich, K.A.; Sanchez-Perez, L.; Archer, G.E.; Schmittling, R.J.; Norberg, P.K.; Herndon, J.E., 2nd; Healy, P.; Congdon, K.L.; et al. Dendritic Cells Enhance Polyfunctionality of Adoptively Transferred T Cells That Target Cytomegalovirus in Glioblastoma. Cancer Res. 2018, 78, 256–264.
- Tran, E.; Nielsen, J.S.; Wick, D.A.; Ng, A.V.; Johnson, L.D.; Nesslinger, N.J.; McMurtrie, E.; Webb, J.R.; Nelson, B.H. Polyfunctional T-cell responses are disrupted by the ovarian cancer ascites environment and only partially restored by clinically relevant cytokines. PLoS ONE 2010, 5, e15625.
- De Groot, R.; Van Loenen, M.M.; Guislain, A.; Nicolet, B.P.; Freen-Van Heeren, J.J.; Verhagen, O.; Van Den Heuvel, M.M.; De Jong, J.; Burger, P.; Van Der Schoot, C.E.; et al. Polyfunctional tumor-reactive T cells are effectively expanded from non-small cell lung cancers, and correlate with an immune-engaged T cell profile. Oncoimmunology 2019, 8, e1648170.
