Azaphenothiazines are the largest and most perspective group of modified phenothiazines, and they exhibit variety of biological activities. The review sums up the current knowledge on the anticancer activity of isomeric pyridobenzothiazines and dipyridothiazines, which are modified azaphenothiazines with one and two pyridine rings, respectively, against 10 types of cancer cell lines. Some 10-substituted dipyridothiazines and even 10-unsubstituted parent compounds, such as 10H-1,9-diazaphenothiazine and 10H-3,6-diazaphenothiazine, exhibited very potent action with the IC50 values less than 1 µg/mL and 1 µM against selected cancer cell lines. The strength of the anticancer action depends both on the tricyclic ring scaffolds and the substituents at the thiazine nitrogen atom.
- pirydobenzothiazines
- dipyridothiazines
- anticancer agents
- gens analysis
- apoptosis
1. Introduction
Phenothiazines are a very important class of heterocyclic compounds possessing a tricyclic dibenzo-[1,4]-thiazine ring system. This system, as the phenothiazinium salt, was for the first time synthesized by Caro in 1876 as a dye called methylene blue (1) [1]. Later in 1883, Bernthsen obtained free 10H-phenothiazine (2) in the reaction of sulfurization of diphenylamine with elemental sulfur [2] (Figure 1). In 1950s, the researchers at Rhône-Poulenc synthesized phenothiazines which exhibited antihistaminic and antipsychotic activities. Since this time, phenothiazines have been essential drugs (at least 100 phenothiazines) for the clinical treatment of psychotic disorders [3], among which chlorpromazine, thioridazine, fluphenazine, promethazine, and trifluoperazine are the most known. Those classical phenothiazines contained the dialkylaminoalkyl substituents at the thiazine nitrogen atom (in position 10) and additionally small substituents in position 2. They exhibit also analgesic, anti-emetic, antihelmentic, and antipuritic activities [3][4].
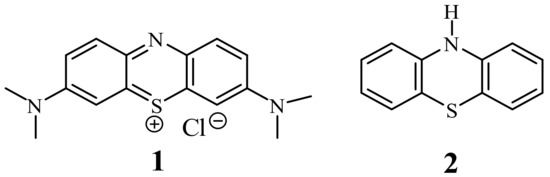
For a few decades, there has been an extremely high interest in searching for new derivatives of phenothiazines possessing different activities. The first approach is called drug repurposing or drug repositioning, and the use of known drugs for new biological targets decreases the cost of research, while maintaining quality and proven safety [5][6][7]. Phenothiazines with thioridazine at the lead are regarded as the most up and coming in a treatment of the multidrug resistant tuberculosis [8][9][10][11], cancer cells [5][6][7][12][13][14][15][16], and neurodegenerative diseases [13]. They also exhibit antiviral, anti-emetic, antipruritic, anti-inflammatory, antitussive, analgesic, antibacterial, and antifungal activities [13][14][17].
The second approach is based on the synthesis of new structurally modified phenothiazines. This modification involves the insertion of new substituents at the nitrogen atom, oxidation of the sulfide group into sulfoxide and sulfone groups in the thiazine ring, and the exchange of one or two benzene rings with other rings (homoaromatic and heteroaromatic). The last modification is the most perspective because it allows the formation of not only a new phenothiazine scaffold (not only tricyclic but also tetracyclic and pentacyclic), but also allows to introduce new substituents. The introduction of the azine ring such as pyridine, pyridazine, pyrimidine, pyrazine, or quinoline in the place of the benzene ring, leads to new derivatives of phenothiazines, azaphenothiazines. Over 50 types of azaphenothiazine scaffolds are known which belong to varied heterocyclic ring systems [18][19].
The anticancer activity of classical and modified phenothiazines was summarized for the first time by Motohashi in the book chapter in the late 1980s [20]. Later, he with his research groups started extensive studies on the anticancer activity of new phenothiazines which were the inspiration to other research groups. They synthesized modified tricyclic phenothiazines with amidoalkyl, sulfonamidoalkyl, and chloroethylureidoalkyl substituents in position 10 and replaced the benzene ring with naphthalene. These phenothiazines exhibited cytotoxic activities against ten different human tumor cell lines: leukemia, melanoma, small cell lung, colon, central nervous system, renal, breast, ovarian, and prostate tumors. Their results were published in over 50 original papers and reviews [21][22][23][24][25][26][27][28][29].
The modified phenothiazines exhibit not only anticancer activity, but a wide range of other activities such as the reversal of multidrug resistance, anti-inflammatory, antibacterial, antiviral, antiplasmid, cholinesterase inhibitory, antioxidant, and antihyperlipidemic and are considered potential agents in the treatment in Alzheimer’s and Creutzfeldt–Jakob diseases, published in hundreds of original papers and patents, and summarized in numerous comprehensive review papers and chapters [18][19][30][31][32][33][34][35][36][37][38].
The modification of the phenothiazine structures with the pyridine ring leads to different pyridobenzothiazines and dipyridothiazines, also named x-monoazaphenothiazines and x,y-diazaphenothiazines, the largest but also most diverse family of azaphenothiazines. The aim of this review is to present comprehensively anticancer activities of those azaphenothiazines.
2. Pyridobenzothiazines
Pyridobenzothiazines represent linearly condensed tricyclic ring systems where the 1,4-thiazine ring is fused with the benzene and pyridine rings along the C-C bonds. As they differ from classical phenothiazines in the presence of the additional nitrogen atom instead of the carbon atom, they are named azaphenothiazines (1-, 2-, 3- and 4-azaphenothiazines).
2.1. 1-Azaphenothiazines (Pyrido[3,2,-b]benzo[1,4]thiazines)
Of tPhe 1-azaphenothiazines, antitumor activity has so far been described only for 10-substituted derivatives including a di are a very important class of heterocyclic compounds possessing a tricyclic dibenzo-[1,4]-thiazine ring system. This system, as the phenothiazinium salt, was for the first time synthesized by Caro in 1876 as a dye called methylaminopropyl groupene blue (31) [1]. Later in 1883, cBernthsen obtalled ined free 10H-prhenothipendyliazine (Figure 22), and a hexyl chain containing amino and nitroin the reaction of sulfurization of diphenylamine with elemental sulfur [2] (Figure 1). In group1950s or a bromine atom at the end (4–9), the researchers at Rhône-Poulenc synthesized phenothiazines which (Figure 3).

Texhibite anticancerd antihistaminic and antipsychotic activity of prothipendyl was investigated recently using the cultured glioblastomaies. Since this time, phenothiazines have been essential drugs (at least 100 phenothiazines) for the clinical treatment of psychotic disorders SNB-19[3], amelanoma C-32, breast cancer MCF-7ong which chlorpromazine, thioridazine, fluphenazine, promethazine, and ductal carcinoma T47D cell lines with the normal human fibroblast (HFF-1) cell line used as a control. The cell lines used showed a different sensitivitytrifluoperazine are the most known. Those classical phenothiazines contained the dialkylaminoalkyl substituents at the thiazine nitrogen atom (in position 10) and additionally small substituents in position 2. They exhibit also analgesic, anti-emetic, antihelmentic, and antipuritic activities t[3][4].

For a few prothipendyl. The MCF-7 and the C32 cells lines were found to be the most sensitive with the IC50decades, there has been an extremely high interest in searching for new derivatives of phenothiazines possessing different activities. The first approach is vcalue of 23.2 µg/mL and 28.1 µg/mL, respectively. The T47D lled drug repurposing or drug repositioning, and the SNB19 cells lines were the most resistant for tuse of known drugs for new biological targets decreases the cost of research, while maintaining quality and proven safety [5][6][7]. Phe notested compound (with IC50 hiazines with thiofridazine 32.3 µg/mL and 36.6 µg/mL, respectively). Prothipendyl was found to be non-toxicat the lead are regarded as the most up and coming in a treatment of the multidrug resistant tuberculosis (IC50[8][9][10][11], >cancer 50cells µg/mL)[5][6][7][12][13][14][15][16], and neurodegainstenerative diseases [13]. tThe normal human fibroblast (HFF-1) cell line in comparison with cisplatin used as a reference (IC50y also exhibit antiviral, anti-emetic, antipruritic, anti-inflammatory, antitussive, analgesic, antibacterial, and =antifungal 8.2activities µg/mL) [4513][4614][4717].
A
The setcond of twenty-one novel 10-substituted 1-azaapproach is based on the synthesis of new structurally modified phenothiazines (4–9). This modificontaining aminohexyl and bromohexylation involves the insertion of new substituents at the nitrogen atom, oxidation of the sulfide groups together with a nitro into sulfoxide and sulfone groups in the pyridthiazine ring (Figure 3), and wthere investigated for their action against six cancer cell lines (malignant brain cancer T98G, lung cancer H460, thyroid cancer SNU80, oral cancer KB, blood cancer THP1, and blood cancer U937) and two normal cell lines (the normal lung fibroblast MRC5 and the normal embryonic kidney cells HEK293) with exchange of one or two benzene rings with other rings (homoaromatic and heteroaromatic). The last modification is the most perspective because it allows the formation of not only a new phenothiazine scaffold (not only tricyclic but also tetracyclic and pentacyclic), but also allows to introduce new substituents. The introduction of the actinomycin D used as the standard drugzine ring such as pyridine, pyridazine, pyrimidine, pyrazine, or quinoline in the studies.
Eplaceven of the compounds tested exhibited distinct inhibitory action against selected cancer lines (IC50 < 10 µg/mL) benzene ring, leads to new derivatives of phenothiazines, azaphenothiazines. Over 50 types of azaphenothiazine scanffold were a few times more effective in comparisons are known which belong to varied heterocyclic ring systems to[18][19].
The a sntandard drug actinomycin D. Compound 6 (wicancer activity of classical and modifith the piperidinylhexyl group) showed the highest activity against the lung cancer H460, the malignant brain cancer T98G,d phenothiazines was summarized for the first time by Motohashi in the book chapter in the late 1980s [20]. Land the thyroid cancer SNU80 with the IC50 valuter, he with his research of 2.27–3.8 µg/mL, respectively. Similarlygroups started extensive studies on the anticancer active were derivativity of new phenothiazines 9 (whith the pyrrolidinyl-piperidinylhexylch were the inspiration to other research group) against the H460 and SNU80 cell lines (IC50 =s. They synthesized modified tricyclic phenothiazines with 2.1 and 2.3 µg/mL)midoalkyl, 7 (sulfonamidorpholinylhexyl group)alkyl, and 5 (pyrchloroethylidinylhexyl) against H460 line (IC50ureidoalkyl substituents in position 10 and replaced the benzene ring =with 2.5 and 2.7 µg/mL). The derivativenaphthalene. These phenothiazines 4exhibited wascytotoxic activeities against those threeen different human tumor cell lines showing the IC50 v: leukemia, melanoma, smallues of 3.8–6.2 µg/mL cell lung, [48].
Verycolon, recently, prothipendyl exhibited antiviral action against a mosquito-transmitting alphavirus (CHIKV) inducing CHIK feverral nervous system, renal, breast, ovarian, and prostate tumors. Their results were published in over 50 original papers and reviews [4921][22][23][24][25][26][27][28][5029].
Ot
Ther derivatives of 1-azamodified phenothiazines exhibited mai not only antihistaminic, anticholinergiccancer activity, but a wide range of other activities such as the reversal of multidrug resistance, anti-emeticinflammatory, antibacterial, antitussive, neuropharmacologicalviral, antiplasmid, cholinesterase inhibitory, antibacterialoxidant, and antitubercular activitiehyperlipidemic and are considered potential agents in the treatment in Alzheimer’s and were the subject of many publications and reviewCreutzfeldt–Jakob diseases, published in hundreds of original papers and patents, and summarized in numerous comprehensive review papers and chapters [5118][5219][5330][5431][5532][5633][5734][5835][5936][6037][61][6238].
2.2. 2-Azaphenothiazines (Pyrido[4,3-b]benzo[1,4]thiazines)
Despite t
The modifact that 10H-2-azaication of the phenothiazines (10) (Figure 4) was syntructhesized in the late 1950s [63][64], theures with the pyridine are no reports in the literature about the antitumor activity of molecules containing the 2-ring leads to different pyridobenzothiazines and dipyridothiazines, also named x-monoazaphenothiazines and x,y-diazaphenothiazine skeleton. Instead of that, the aminoalkyl derivatives exhibited antipsychotic and sedative properties s, the largest but also most diverse family of azaphenothiazines. The aim of this review is to present comprehensively anticancer activities of those azaphenothiazines.
2. Pyridobenzothiazines
2.1. 1-Azaphenothiazines (Pyrido[3,2,-b]benzo[1,4]thiazines)


2.2. 2-Azaphenothiazines (Pyrido[4,3-b]benzo[1,4]thiazines)
[65]
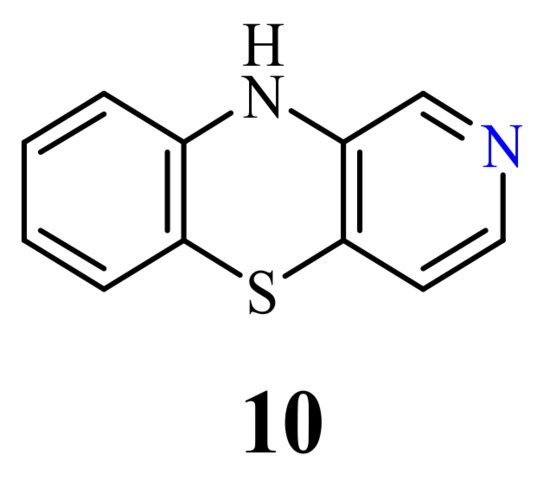
2.3. 3-Azaphenothiazines (Pyrido[3,4-b]benzo[1,4]thiazines)
H
11
[66]
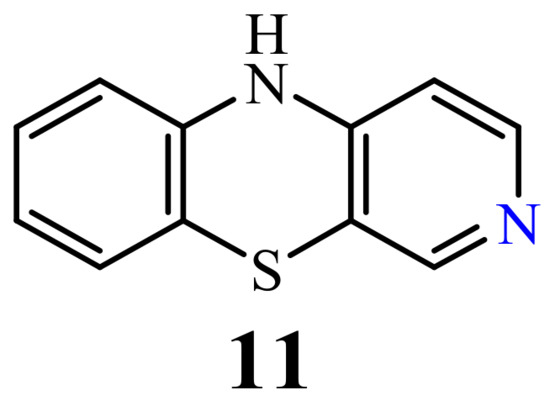
2.4. 4-Azaphenothiazines (Pyrido[2,3-b]benzo[1,4]thiazines)
H
12
[67]
[68]

| Investigated Compounds | Substituents at the Thiazine Nitrogen Atom | Activity, IC50 [Ref] | Cancer Cell Lines |
|---|---|---|---|
| Pyridobenzothiazines | |||
| 1-Azaphenothiazine 3 |
 |
23.2 µg/mL [45][46][47] 28.1 µg/mL 32.3 µg/mL 36.6 µg/mL |
C-32 MCF-7 T47D SNB-19 |
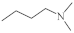
| 4 |  |
3.8–6.2 µg/mL [48] | H460,T98G, SNU80 |
| 6 | 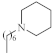 |
2.27–3.8 µg/mL [48] | H460, T98G, SNU80 |
| 7 | 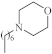 |
2.5 µg/mL [48] | H460 |
| 9 |  |
2.1–2.3 µg/mL [48] | H460, SNU80 |
| Dipyridothiazines | |||
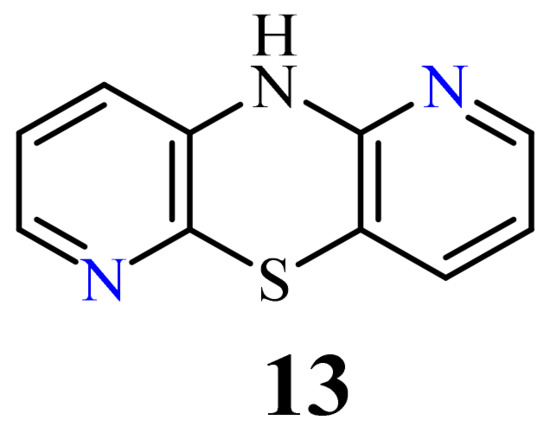
| 1,6-Diazaphenothiazine 13 | H | 4.8 µg/mL [47] 7.5 µg/mL |
MCF-7 C-32 |
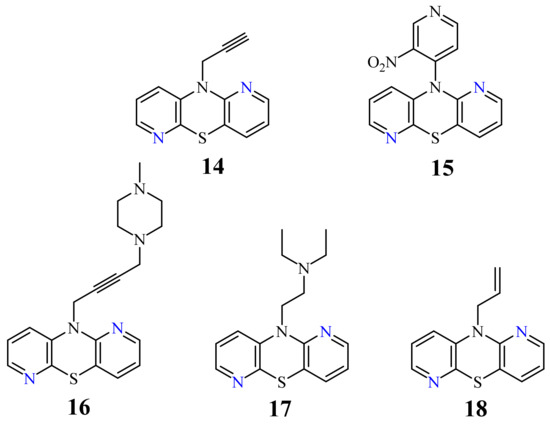
| 14 |  |
3.9 µg/mL [47] | MCF-7 |
| 15 | 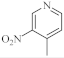 |
4.6 µg/mL [47] | MCF-7 |
| 16 | 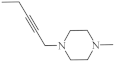 |
7.5 µg/mL [47] | MCF-7 |
| 17 | 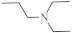 |
6.6 µg/mL [47] | C-32 |
| 18 |  |
18.9 µg/mL [47] | SNB-19 |
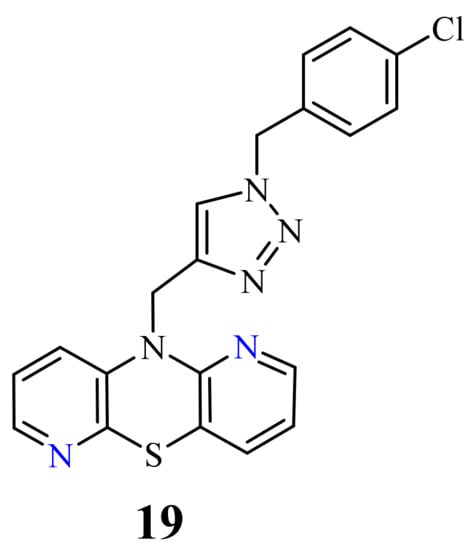
| 19 | 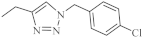 |
0.25-4.66 µM [69] | SNB-19, Caco-2, A549, MDA-MB231 |
| 1,8-Diazaphenothiazine | |||
| 20 | H | 5 µg/mL [70][71] 10 µg/mL |
SW-948 L-1210. |
| 23 | 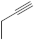 |
26-46 µg/mL [70] | SNB-19, C-32, T47D |
| 24 | 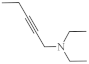 |
26.1 µg/mL [70] | C-32 |
| 25 |  |
1.82 µM [69] | A549 |
| 1,9-Diazaphenothiazine | |||
| 26 | H | 3.83 µM [72] | C-32 |
| 27 | 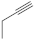 |
3.85 µM [72] 3.37 µM |
SNB-19 C-32 |
| 28 | 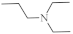 |
0.34 µM [72] 2.13 µM |
SNB-19 MDA-MB-321 |
| 2,7-Diazaphenothiazine | |||
| 29 | H | 0.3 µg/mL [73] 1.7 µg/mL 2.4 µg/mL 3.6 µg/mL 3.1 µg/mL 3.9 µg/mL 5.5 µg/mL 4.1 µg/mL 5.9 µg/mL 6.5 µg/mL 6.8 µg/mL 7.1 µg/mL 8.4 µg/mL |
HOP-62 HOP-92 colon 205 HTC-116 RXF 393 736-0 ACHN HL-60(TB) HS 578T M-14SF-539, SNB-19 OVCAR-8 PC-3 |
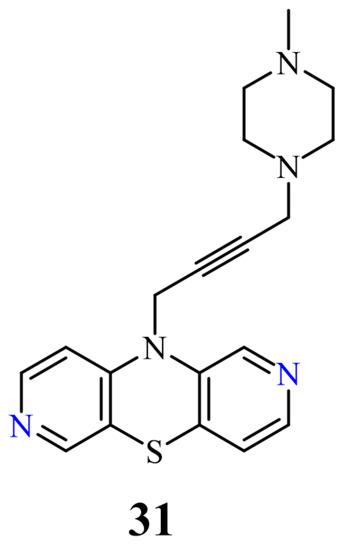
| 31 | 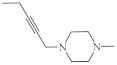 |
9.6 µg/mL [45] | T-47D |
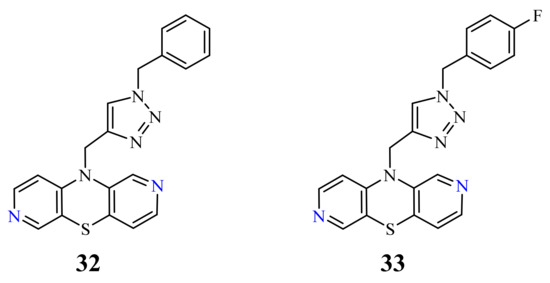
| 32 | 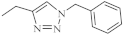 |
0.26 µM [69] 0.77 µM |
Caco-2, A549 MDA-MB231 |
| 33 | 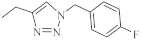 |
0.64 µM [69] 0.65 µM |
MDA-MB231 A549 |
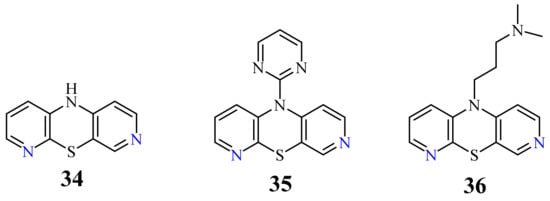
| 3,6-Diazaphenothiazine | |||
| 34 | H | 0.46 µg/mL [46] 0.72 µg/mL 0.62 µM [74] |
SNB-19 C-32, MCF-7 A2780 |
| 35 | 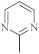 |
0.73 µg/mL [46] | MCF-7 |
| 36 |  |
6.3 µg/mL [46] 11.3 µg/mL |
C-32 MCF-7 |
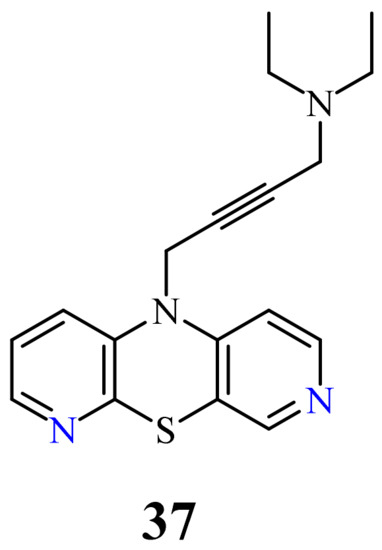
| 37 |  |
0.11 µg/mL [75] | SNB-19 |
| 38 | 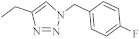 |
0.25 µM [69] | Caco-2, A549 |
3. Dipyridothiazines
Dipyridothiazines mean linearly condensed tricyclic ring systems where the 1,4-thiazine ring is fused with two pyridine rings along the C-C bonds. As they contain two additional nitrogen atoms (instead of two carbon atoms) in comparison with classical phenothiazines, they are named diazaphenothiazines. Out of ten theoretically possible dipyridothiazine systems, only six have been known as 1,6-, 1,8-, 1,9-, 2,7-, 3,6-, and 3,7-diazaphenothiazines. This small amount of well-known dipyridothiazine types is a result of the difficulties in their synthesis involving orto disubstituted (2,3- and 3,4-)pyridines
[19]
.
3.1. 1,6-Diazaphenothiazines (Dipyrido[2,3-b;2’,3’-e][1,4]thiazines)
H
13
[76]
[47]
50
H
13
14
15
50
16
H
13
17
18
50
50
[47]
[77]
p
19
p
50
[69]
50
H3
TP53
CDKNIA
BCL-2
BAX
H3
TP53
CDKN1A
BCL-2
H3
TP53
CDKN1A
BCL-2
BAX
[69]
3.2. 1,8-Diazaphenothiazines (Dipyrido[2,3-b;3’,4’-e][1,4]thiazines)
H
20
20
21
20
22
22
20
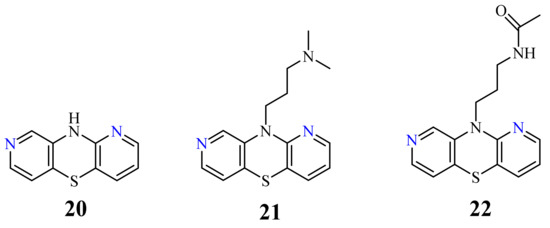
23
50
24
50
[45]
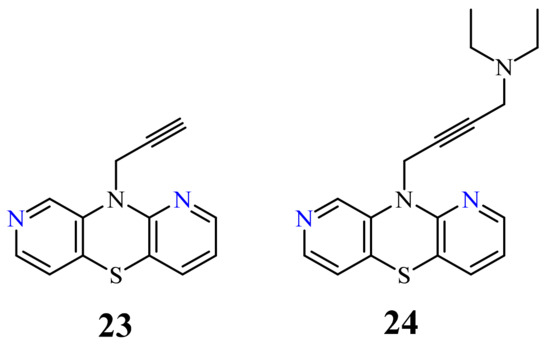
[78]
50
25
p
50
19
50
[69]
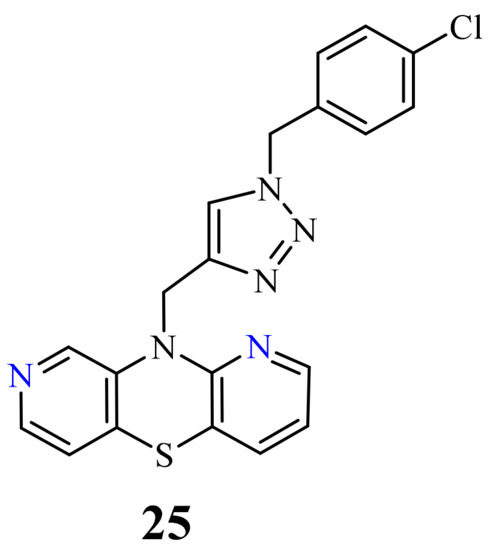
3.3. 1,9-Diazaphenothiazines (Dipyrido[2,3-b;2’,3’-e][1,4]thiazines).
H
26
[79]
H
26
[72]
H
26
50
50
27
28

27
50
50
28
50
50
H3
TP53
CDKN1A
BCL-2
BAX
H3
TP53
CDKN1A
BAX/BCL-2
[72]
3.4. 2,7-Diazaphenothiazines (Dipyrido[3,4-b;3’,4’-e][1,4]thiazines)
H
29
30
[82]
H
29
29
50
29
50
H
29
50
[84]
31
50
50
31
TP53, CDKN1A, BCL-2
BAX
CDKN1A
BCL-2/BAX
[45]
50
32
50
33
p
50
50
[69]
3.5. 3,6-Diazaphenothiazines (Dipyrido[2,3-b;4’,3’-e][1,4]thiazines)
[46]
H
34
34
50
35
50
36
[46]
H
34
50
34
H
34
[74]
37
50
H3
TP53
CDKN1A
BCL-2
BAX
[75]
p
38
p
50
[69]
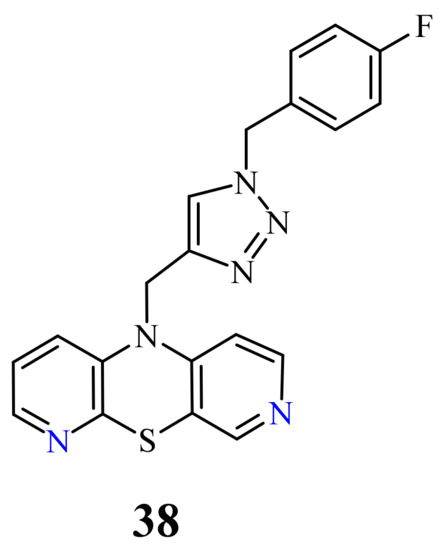
[83]
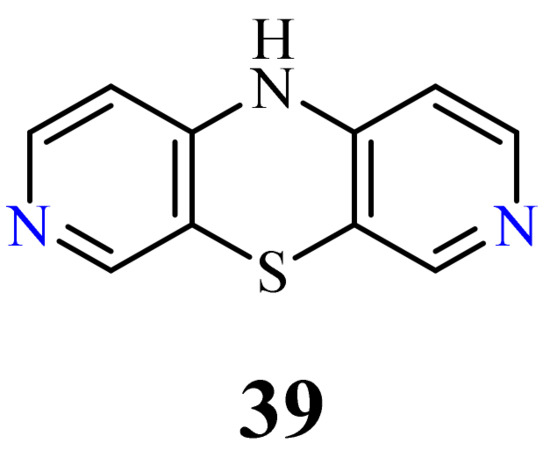
3.6. 3,7-Diazaphenothiazines (Dipyrido[3,4-b;4’,3’-e][1,4]thiazines)
This group of diazaphenothiazines was described in the 1960s
3.6. 3,7-Diazaphenothiazines (Dipyrido[3,4-b;4’,3’-e][1,4]thiazines)
This group of diazaphenothiazines was described in the 1960s
. The parent compound 10
H
-3,7-diazaphenothiazine (
39
) and its 10-derivatives containing the diethylaminoethyl and dimethylaminopropyl substituents (
) exerted an antihistaminic action. Unfortunately, these activities were not substantially better than those of the leading phenothiazine drugs in the pharmaceutical market at the time. The anticancer activity of these compounds has not yet been evaluated.
Out of the six types of isomeric dipyridothiazines, five types were tested successfully against various kinds of cancer cell lines.
Most of the biological results discussed in this chapter were obtained by the authors in cooperation with international and domestic research groups.
The information provided on anticancer activity of pyridobenzothiazines and dipyridothiazines was summarized in
.
References
- Reinhardt, C.; Travis, A.S. Heinrich Caro and the Creation of Modem Chemical Industry, Chemists and Chemistry; Springer Science + Business Media: Dordrecht, The Netherlands, 1998; Volume 19, pp. 242–245.
- Bernthsen, A. Ű̈ber das Methylenblau. Ber. Dtsch. Chem. Ges. 1883, 16, 2896–2904.
- Gupta, R.R.; Kumar, M. Synthesis, properties and reactions of phenothiazines. In Phenothiazine and 1,4-Benzothiazines—Chemical and Biological Aspect; Gupta, R.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 1–161.
- Ohlow, M.J.; Moosmann, B. Foundation review: Phenothiazine: The seven lives of pharmacology’s first lead structure. Drug Discov. Today 2011, 16, 119–131.
- Huang, J.; Zhao, D.; Liu, Z.; Liu, F. Repurposing psychiatric drugs as anti-cancer agents. Cancer Lett. 2018, 419, 257–265.
- Kang, S.; Lee, J.M.; Jeon, B.; Elkamhawy, A.; Paik, S.; Hong, J.; Oh, S.-J.; Paek, S.H.; Lee, C.J.; Hassan, A.H.E.; et al. Repositioning of the antipsychotic trifluoperazine: Synthesis, biological evaluation and in silico study of trifluoperazine analogs as anti-glioblastoma agents. Eur. J. Med. Chem. 2018, 151, 186–198.
- Wu, C.-H.; Bai, L.-Y.; Tsai, M.-H.; Chu, P.-C.; Chiu, C.-F.; Chen, M.Y.; Chiu, S.-J.; Chiang, J.-H.; Weng, J.-R. Pharmacological exploitation of the phenothiazine antipsychotics to develop novel antitumor agents—A drug repurposing strategy. Sci. Rep. 2016, 6, 1–16.
- Kristiansen, J.E.; Dastidar, S.G.; Palchoudhuri, S.; Roy, D.S.; Das, S.; Hendricks, O.; Christensen, J.B. Phenothiazines as a solution for multidrug resistant tuberculosis: From the origin to present. Int. Microb. 2015, 18, 1–12.
- Amaral, L.; Viveiros, M. Thioridazine: A non-antibiotic drug highly effective, in combination with first line anti-tuberculosis drugs, against any form of antibiotic resistance of mycobacterium tuberculosis due to its multi-mechanisms of action. Antibiotics 2017, 6, 1.
- Sharma, S.; Singh, A. Phenothiazines as anti-tubercular agents: Mechanistic insights and clinical implications. Expert Opin. Investig. Drugs 2011, 20, 1665–1676.
- Sellamuthu, S.; Bhat, M.F.; Kumar, A.; Singh, S.K. Phenothiazine: A better scaffold against tuberculosis. Mini-Rev. Med. Chem. 2017, 17, 1442–1451.
- Spengler, G.; Csonka1, Á.; Molnár, J.; Amaral, L. The anticancer activity of the old neuroleptic phenothiazine-type drug thioridazine. Anticancer Res. 2016, 36, 5701–5706.
- Varga, B.; Csonka, Á.; Csonka, A.; Molnár, J.; Amaral, L.; Spengler, G. Possible biological and clinical applications of phenothiazines. Anticancer Res. 2017, 37, 5983–5993.
- Sudeshna, G.; Parimal, K. Muliple non-psychiatric effect of phenothiazines: A review. Eur. J. Pharmacol. 2010, 648, 6–14.
- Yue, H.; Huang, D.; Qin, L.; Zheng, Z.; Hua, L.; Wang, G.; Huang, J.; Huang, H. Targeting lung cancer stem cells with antipsychological drug thioridazine. BioMed Res. Int. 2016.
- Nagy, S.; Argyelan, G.; Molnár, J.; Kawase, M.; Motohashi, N. Antitumor activity of phenothiazine-related compounds. Anticancer Res. 1996, 16, 1915–1918.
- Mosnaim, A.D.; Ranade, V.V.; Wolf, M.E.; Puente, J.; Valenzuela, M.A. Phenothiazine molecule provides the basic chemical structure for various classes of pharmacotherapeutic agents. Am. J. Therapeut. 2006, 13, 261–273.
- Pluta, K.; Jeleń, M.; Morak-Młodawska, B.; Zimecki, M.; Artym, J.; Kocięba, M.; Zaczyńska, E. Azaphenothiazines a promising phenothiazine derivatives. An insight into nomenclature, synthesis, structure elucidation and biological properties. Eur. J. Med. Chem. 2017, 138, 774–806.
- Pluta, K.; Morak-Młodawska, B.; Jeleń, M. Synthesis and properties of diaza-, triaza- and tetraazaphenothiazines. J. Heterocycl. Chem. 2009, 46, 355–391.
- Motohashi, N. Antitumor activity of phenothiazines (phenothiazine oncology). In Phenothiazines and 1,4-Benzothiazines. Chemical and Biological Aspects; Gupta, R.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 705–770.
- Sakagami, H.; Takahashi, H.; Yoshida, H.; Yamamura, M.; Fukuchi, K.; Gomi, K.; Motohashi, N.; Takeda, M. Induction of DNA fragmentation in human Myelogenous Leukaemic cell lines by phenothiazine-related compounds. Anticancer Res. 1995, 15, 2533–2540.
- Motohashi, N.; Sakagami, H.; Kamata, K.; Yamamoto, Y. Cytotoxicity and differentiation-inducing activity of phenothiazine and benzo[a]phenothiazine derivatives. Anticancer Res. 1991, 11, 1933–1937.
- Wuonola, M.A.; Palfreyman, M.G.; Motohashi, N.; Kawase, M.; Gabay, S.; Gupta, R.R.; Molnár, J. The primary in vitro anticancer activity of “half-mustard type” phenothiazines in NCI’s revised anticancer screening paradigm. Anticancer Res. 1998, 18, 337–348.
- Motohashi, N.; Kurihara, T.; Sakagami, H.; Szabo, D.; Csuri, K.; Molnár, J. Chemical structure and tumor type specificity of “half-mustard type” phenothiazines. Anticancer Res. 1999, 19, 1859–1864.
- Motohashi, N.; Kawase, M.; Kurihara, T. Synthesis and antitumor activity of 1-[2(chloroethyl)-3-(-substituted-10H-phenothiazin-10-yl)]alkyl-1-ureas as potent anticancer agents. Anticancer Res. 1996, 16, 2525–2532.
- Motohashi, N.; Kawase, M.; Saito, S. Synthesis and biological activity of N-acylphenothiazines. Int. J. Antimicrob. Agents. 2000, 14, 203–207.
- Motohashi, N.; Kawase, M.; Saito, S.; Sakagami, H. Antitumor potential and possible targets of phenothiazine-related compounds. Curr. Drug Targets 2000, 1, 237–245.
- Motohashi, N.; Kawase, M.; Satoh, K.; Sakagami, H. Cytotoxic potential of phenothiazines. Curr. Drug Targets 2006, 7, 1055–1066.
- Gaye-Seye, M.D.; Aaron, J.J.; Parkanyi, C.; Motohashi, N. Luminescence and photophysical properties of benzo[a]phenothiazines-therapeutic, physico-chemical and analytical applications. Curr. Drug Targets 2006, 7, 1083–1093.
- Bisi, A.; Meli, M.; Gobbi, S.; Rampa, A.; Tolomeo, M.; Dusonchet, L. Multidrug resistance reverting activity and antitumor profile of new phenothiazine derivatives. Bioorg. Med. Chem. 2008, 16, 6474–6482.
- Khandelwal, N.; Yadav, A.; Gautam, N.; Gautam, D.C. Study and synthesis of biologically active phenothiazines, their sulfones, and ribofuranosides. Nucleos. Nucleot. Nucl. Acids 2012, 31, 680–691.
- Gautam, N.; Garg, A.; Lal, T.; Gautam, D.C.; Joshi, J. Synthesis and antimicrobial assessment of new substituted 10H-phenothiazines, their sulfone derivatives, and ribofuranosides. Heterocycl. Commun. 2014, 20, 343–349.
- Prinz, H.; Ridder, A.-K.; Vogel, K.; Böhm, K.J.; Ivanov, I.; Ghasemi, J.B.; Aghaee, E.; Müller, K. N-Heterocyclic (4-phenylpiperazin-1-yl)methanones derived from phenoxazine and phenothiazine as highly potent inhibitors of tubulin polymerization. J. Med. Chem. 2017, 60, 749–766.
- Zhang, J.-X.; Guo, J.-M.; Zhang, T.-T.; Lin, H.-J.; Qi, N.-S.; Li, Z.-G.; Zhou, J.-C.; Zhang, Z.-Z. Antiproliferative phenothiazine hybrids as novel apoptosis inducers against MCF-7 breast cancer. Molecules 2018, 23, 1288.
- Krishnan, K.G.; Kumar, C.U.; Lim, W.-M.; Mai, C.-W.; Thanikachalam, P.V.; Ramalingan, C. Novel cyanoacetamide integrated phenothiazines: Synthesis, characterization, computational studies and in vitro antioxidant and anticancer evaluations. J. Mol. Struct. 2020, 1199, 127037.
- Pluta, K.; Morak-Młodawska, B.; Jeleń, M. Recent progress in biological activities of synthesized phenothiazines. Eur. J. Med. Chem. 2011, 46, 3179–3189.
- Gopi, C.; Dhanaraju, M.D. Recent progress in synthesis, structure and biological activities of phenothiazine derivatives. Rev. J. Chem. 2019, 9, 95–126.
- Gao, Y.; Sun, T.-Y.; Bai, W.-F.; Bai, C.-G. Design, synthesis and evaluation of novel phenothiazine derivatives as inhibitors of breast cancer stem cells. Eur. J. Med. Chem. 2019, 183, 111692.
- Yale, H.L.; Bernstein, J. Azaphenothiazine Compound and Their Preparation. U.S. Patent 2943086, 6 May 1960.
- Uhrig, S.; Hirth, N.; Broccoli, L.; Von Wilmsdorff, M.; Bauer, M.; Sommer, C.; Zink, M.; Steiner, J.; Frodl, T.; Malchow, B.; et al. Reduced oxytocin receptor gene expression and binding sites in different brain regions in schizophrenia: A post-mortem study. Schizophr. Res. 2016, 177, 59–66.
- Winkler, D.; Pjrek, E.; Lanzenberger, R.; Baldinger, P.; Eitel, D.; Kasper, S.; Frey, R. Actigraphy in patients with treatment-resistant depression undergoing electroconvulsive therapy. J. Psychiatr. Res. 2014, 57, 96–100.
- Kleinmann, A.; Schrader, V.; Stübner, S.; Greil, W.; Kahl, K.G.; Bleich, S.; Grohmann, R.; Frieling, H.; Toto, S. Psychopharmacological treatment of 1650 in-patients with acute mania-data from the AMSP study. J. Affect. Disord. 2016, 191, 164–171.
- Scharfetter, J.; Fischer, P. QTc prolongation induced by intravenous sedation with Haloperidol, Prothipendyl and Lorazepam. Neuropsychiatrie 2014, 28, 1–5.
- Declercq, T.; Petrovic, M.; Azermani, M.; Vander Stichle, R.; De Sutter, A.I.; Van Driel, M.L.; Christiaens, T. Withdrawal versous continuation of chronic antipsychotic drugs for behavioral and psycholofical symptoms in older people with dementia. Cochrane Database Syst. Rev. 2013, 28, 1–95.
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D. Synthesis and anticancer and lipophilic properties of 10-dialkylaminobutynyl derivatives of 1,8- and 2,7-diazaphenothiazines. J. Enzym. Inhib. Med. Chem. 2016, 31, 1132–1138.
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Suwińska, K.; Jeleń, M.; Kuśmierz, D. 3,6-Diazaphenothiazines as potential lead molecules—Synthesis, characterization and anticancer activity. J. Enzym. Inhib. Med. Chem. 2016, 31, 1512–1519.
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M. Synthesis, spectroscopic characterization, and anticancer activity of new 10-substituted 1,6-diazaphenothiazines. Med. Chem. Res. 2016, 25, 2425–2433.
- Kushwaha, K.; Kaushik, N.K.; Kaushik, N.; Chand, M.; Kaushik, R.; Ha Choi, E.; Jain, S.C. Novel aminoalkylated azaphenothiazines as potential inhibitors of T98G, H460 and SNU80 cancer cell lines in vitro. Bioorg. Med. Chem. Lett. 2016, 26, 2237–2244.
- Kaur, P.; Hann Chu, J.J. Chikungunya virus: An update on antiviral development and challenges. Drug Discov. Today 2013, 18, 969–983.
- Hagerdorn, H.W.; Zuck, S.; Schulz, R. Prothipendyl: Detection and elimination in the horse—A casereport. Dtsch Tierarztl Wochenschr 1996, 103, 125–127.
- Martindale, the Extra Pharmacopoeia, 29th ed.; Reynolds, J.E.F. (Ed.) Pharmaceutical Press: London, UK, 1989.
- Shaikh, S.M.T.; Seetharamappa, J.; Kandagal, P.B.; Ashoka, S. Binding of the bioactive component isothipendyl hydrochloride with bovine serum albumin. J. Mol. Struct. 2006, 786, 46–52.
- Moreau, A.; Dompmartin, A.; Dubreuil, A.; Leroy, D. Phototoxic and photoprotective effects of topical isothipendyl. Photodermatol. Photoimmunol. Photomed. 1995, 11, 50–54.
- Bibas, N.; Sartor, V.; Bulai Livideanu, C.; Bagheri, H.; Nougue, J.; Giordano- Labadie, F.; Maza, A.; Paul, C.; Chouini-Lalanne, N.; Marguery, M.C. Contact photoallergy to isothipendyl chlorohydrate. Dermatology 2012, 224, 289–291.
- Amin, A.S.; El-Sheikh, R.; Zahran, F.; El-fetough Gouda, A.A. Spectrophotometric determination of pipazethate HCl, dextromethorphan HBr and drotaverine HCl in their pharmaceutical preparations. Spectrochim. Acta A 2007, 67, 1088–1093.
- Atkinson, E.R.; Russ, P.L.; Tucker, M.T. Neuropharmacological profile of 1-azaphenothiazine-10-thiolcarboxylates. J. Med. Chem. 1971, 14, 1005–1007.
- Sharma, A.; Tyagi, E. Synthesis of some substituted pyrido[3,2-b][1,4]benzothiazines and their antibacterial activity. Pharmazie 1991, 46, 746–747.
- Swati, S.S.; Mishira, A.K.; Prakash, L. Synthesis of some novel 1-azaphenothiazines and their mesoionic as analogues of popent CNS-depressants. Phosphorus Sulfur Silicon Relat. Elem. 1996, 117, 111–120.
- Agrawal, H.; Yador, A.K.; Prakash, L. An elegant synthesis of some new potential biologically active pyrido[3,3-b][1,4]benzothiazine derivatives and their nucleosides by phase transfer catalysis. Heterocycl. Commun. 1998, 4, 589–594.
- Kumar, N.; Singh, G.; Khatoon, S.; Yadav, A. Synthesis and antimicrobial activities of novel 10H-pyrido[3,2-b][1,4]benzo[b]thiazine ribofuranosides. Indian J. Chem. B 2003, 42, 2015–2018.
- Madrid, P.; Polgar, W.; Toll, L.; Tanga, M. Synthesis and antitubercular activity of phenothiazines with reduced binding to dopamine and serotonin receptors. Bioorg. Med. Chem. Lett. 2007, 17, 3014–3017.
- Saggiomo, A.; Craig, P.; Gordon, M. Synthesis of 2-aza- and 8-chloro-2-aza-phenothiazine. J. Org. Chem. 1958, 23, 1906–1909.
- Okafor, C.O. Studies in the heterocyclic series. III. The Chemistry of azaphenothiazine compounds. Int. J. Sulfur Chem. B 1971, 6, 239–265.
- Zirkle, C.L.; Kaiser, C. Antipsychotic agents (tricyclic), In Psychopharmacological Agents; Gordon, M., Ed.; Academic Press: New York, NY, USA, 1974; Volume 3, pp. 39–128.
- Clarke, F.H.; Silverman, G.B.; Watnick, C.M.; Sperber, N. 3-Azaphenothiazine and dialkylaminoalkyl derivatives. J. Org. Chem. 1961, 26, 1126–1132.
- Chorvat, R.J.; Desai, B.N.; Radak, S.E.; Bloss, J.; Hirsch, J.; Tenen, S. Synthesis, benzodiazepine receptor binding, and anticonvulsant activity of 2,3-dihydro-3-oxo-5H-pyrido[3,4-b][1,4]benzothiazine-4-carbonitriles. J. Med. Chem. 1983, 26, 845–850.
- Rhone-Poulenc, Azaphenothiazines and Intermediates. British Patent 791190, 26 February 1958.
- Yamada, K.; Miyamoto, M.; Tatsuya, T.; Sato, K.; Soejima, M.; Sato, T.; Kikuchi, K.; Yoshimura, H.; Moriya, K.; Sakuma, Y. Preparation of Heterocycle-Fused Benzothiazine Derivatives as Allergy Inhibitors. Japan Patent WO 9943683 A1 19990909, 2 September 1999.
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D. Design, Synthesis, and Structural Characterization of Novel Diazaphenothiazines with 1,2,3-Triazole Substituents as Promising Antiproliferative Agents. Molecules 2019, 24, 4388.
- Morak-Młodawska, B.; Pluta, K.; Zimecki, M.; Jeleń, M.; Artym, J.; Kocięba, M. Synthesis and selected immunological properties of 10-substituted 1, 8-diazaphenothiazines. Med. Chem. Res. 2015, 24.
- Pluta, K.; Morak-Młodawska, B.; Zimecki, M.; Jeleń, M.; Artym, J.; Kocięba, M. 10H-1,8-Diazaphenothiazine, Its 10-Substituted Derivatives, Their Usage, the Way of Synthesis and Their Pharmaceutical Compositions. Polish Patent PL 227918 B1, 10 July 2013.
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D.; Suwińska, K.; Shkurenko, A.; Czuba, Z.; Jurzak, M. 10H-1,9-diazaphenothiazine and its 10-derivatives: Synthesis, characterisation and biological evaluation as potential anticancer agents. J. Enzyme Inhib. Med. Chem. 2019, 34, 1298–1306.
- Pluta, K.; Jeleń, M.; Morak-Młodawska, B.; Zimecki, M.; Artym, J.; Kocięba, M. Anticancer activity of newly synthesized azaphenothiazines in NCI’s anticancer screening. Pharmacol. Rep. 2010, 62, 319–332.
- Zhang, J.; Chen, M.; Wenzhi, Z.; Okechukwu, P.N.; Morak-Młodawska, B.; Pluta, K.; Jeleń, M.; Md Akim, A.; Ang, K.-P.; Ooi, K.K. 10H-3,6-Diazaphenothiazines Induce G2/M Phase Cell Cycle Arrest, Caspase-dependent Apoptosis and Inhibits Cell Invasion of A2780 Ovarian Carcinoma Cells through Regulation on NF-κB and [BIRC6-XIAP] Complexes. Drug Des. Dev. Ther. 2017, 11, 3045–3063.
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D. Synthesis, anticancer activity and apoptosis induction of novel 3,6-diazaphenothiazines. Molecules 2019, 24, 267.
- Rodig, O.; Collier, R.; Schlatzer, R. Pyridine chemistry. II. Further studies on the Smiles rearrangement of the 3-amino-2,2‘-dipyridyl sulfide system. The synthesis of some 1,6-diazaphenothiazines. J. Med. Chem. 1965, 9, 116–120.
- Morak-Młodawska, B.; Pluta, K.; Jeleń, M. Lipophilicity of New Anticancer 1,6- and 3,6-diazaphenothiazines by of Use RP TLC and Different Computational Methods. J. Chrom. Sci. 2018, 1–6.
- Morak-Młodawska, B.; Pluta, K.; Jeleń, M. Estimation of the Lipophilicity of New Anticancer and Immunosuppressive 1,8-Diazaphenothiazine Derivatives. J. Chrom. Sci. 2015, 53, 462–466.
- Rath, S. Dimethylaminopropyldipyridothiazine. U.S. Patent 2,789,978, 23 April 1957.
- Morak, B.; Pluta, K.; Suwińska, K. Unexpected simple route to novel dipyrido-1,4-thiazine system. Heterocyclic Commun. 2002, 8, 331–334.
- Morak, B.; Pluta, K. Synthesis of novel dipyrido-1,4-thiazines. Heterocycles 2007, 71, 1347–1361.
- Zimecki, M.; Artym, J.; Kocięba, M.; Pluta, K.; Morak-Młodawska, B.; Jeleń, M. The immunosupressive activities of newly synthesized azaphenothiazines in human and mouse models. Cell. Mol. Biol. Lett. 2009, 14, 622–635.
- Pluta, K.; Jeleń, M.; Morak-Młodawska, B. Anticancer activity of selected dipyridothiazines and diquinothiazines determined in National Cancer Institute, in Bethesdzie, USA. Farm. Przegląd Nauk. 2009, 10, 26–29.
- Morak-Młodawska, B.; Pluta, K.; Matralis, A.N.; Kourounakis, A.P. Antioxidant activity of newly synthesized 2,7-diazaphenothiazines. Archiv. Pharm. Chem. Life Sci. 2010, 343, 268–273.
- Morak-Młodawska, B.; Pluta, K.; Jeleń, M. Evaluation of the Lipophilicity of New Anticancer 1,2,3-Triazole-Dipyridothiazine Hybrids Using RP TLC and Different Computational Methods. Processes 2020, 8, 858.
- Okafor, C. Studies in heterocyclic series. I. A novel diazaphenothiazine system. J. Org. Chem. 1967, 32, 2006–2007.
- Okafor, C. Heterocyclic series. II. 3,6-Diazaphenothiazine sulfoxides and other potential antiparasitic and pesticidal agents. J. Chem. Eng. Data 1971, 16, 244–246.
- Kopp, E.; Strell, M. Űber 2,7-diazaphenothiazin. Reaktionen in der Pyridinreihe, IV. Arch. Pharm. 1962, 295, 99–106.
- Strell, M.; Kopp, E.; Janson, R. Verfahren zur Herstellung von 2,7-Diazaphenothiazinen. German Patent DE 1 147 235, 18 April 1963.
- Kopp, E. Tautomerie an einem 2, 7‐Diazaphenothiazinderivat. Reaktionen in der Pyridinreihe, V. Arch. Pharm. 1962, 295, 561–564.
- Werle, E.; Kopp, E.; Leysath, G. Arzneim-Forsch Die Antihistaminwirkung von 2,7-Diazaphenothiazin und einiger seiner derivate. Arzneim-Forsch 1962, 4, 443–444.
