γ-Secretase is an aspartyl protease.。
- protease
- amyloid
- Alzheimer’s disease
- inhibitors
- modulators
1. γ-Secretase Inhibitors: Therapeutic Potential
The search for γ-secretase inhibitors (GSIs) as potential therapeutics for AD had gone on for over two decades, ever since Aβ was discovered to be a normally secreted peptide produced from a variety of cell types in culture [1][67]. Such inhibitors were identified even before the components of the protease were known and ultimately served as critical tools for discovery of presenilin as the catalytic component [2][3][28,29], as described earlier. Initially, these inhibitors were simple peptidomimetics (e.g., TSAs), but pharmaceutical companies quickly developed compounds with much better drug-like properties that allowed in vivo testing for the ability to lower Aβ in the brains of transgenic AD mice (e.g., expressing FAD-mutant APP and presenilin).
Acute treatment with GSIs did show such proof of principle for these drug candidates [4][5][68,69]; however, chronic treatment revealed serious peripheral toxicities [6][7][70,71], such as gastrointestinal bleeding, immunosuppression, and skin lesions, all effects that could be traced to inhibition of Notch proteolysis and signaling. As AD patients would be required to take GSIs for years and perhaps decades, the severe toxic consequences of γ-secretase inhibition caused great concern. While there were hopes for a therapeutic window that would allow lowering brain Aβ levels without the peripheral Notch-deficient toxicity, the failure of one GSI, semagacestat (Figure 19), in phase III clinical trials, dashed these hopes [8][72]. The trial resulted in unacceptable peripheral toxicities and—more worrisome—cognition that was worse than the placebo control groups.
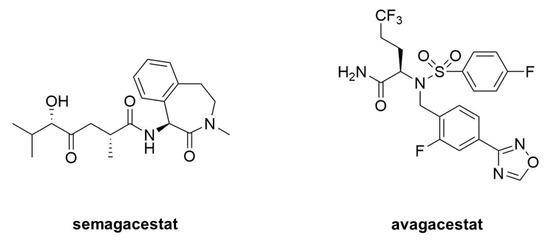
Figure 19. Clinical candidate γ-secretase inhibitors (GSIs). Semagacestat (left) is nonselective for APP vis-à-vis Notch, while avagacestat (right) is reported as selective for blocking γ-secretase proteolysis of APP over Notch.
Because all the serious toxic effects were apparently caused by inhibition of Notch signaling, the focus then went toward finding GSIs that could selectively inhibit the proteolysis of APP by γ-secretase without affecting Notch1 proteolysis. This led to the discovery of so-called “Notch-sparing” GSIs [9][10][11][12][73,74,75,76], a misleading term, as these compounds show APP/Notch selectivity and not complete lack of effect on Notch proteolysis. Moreover, the degree of selectivity was a matter of debate, with some reports of a lack of any selectivity for APP [13][14][77,78]. One such compound, avagacestat (Figure 9), went as far as phase II clinical trials, and like semagacestat caused Notch-deficient toxicities at higher doses, equivocal Aβ lowering in cerebrospinal fluid at lower doses, and worsening of cognition [15][79]. Evidence from mouse models suggest that the cognitive worsening may be due to increased γ-secretase substrates [16][80], although elevation of total Aβ, seen in plasma at low inhibitor concentrations, may be responsible [17][18][81,82]. These findings have effectively halted further development of GSIs for AD. Interestingly though, these compounds may be repurposed for oncology, for the treatment of various cancers that involve overactive Notch signaling [19][83].
2. γ-Secretase Modulators: Therapeutic Potential
While GSIs are out of further consideration for AD therapeutics, γ-secretase modulators (GSMs) are still of keen interest [20][84]. These compounds (see Figure 210 for examples) have the effect of lowering Aβ42 levels without decreasing overall Aβ levels or otherwise inhibiting general γ-secretase activity [21][22][85,86]. The decrease in Aβ42 is correlated with an increase in Aβ38, thereby replacing a highly aggregation-prone form of Aβ with a much more soluble form. Thus, these compounds can prevent the formation of plaques and other higher-order assembly states of Aβ42 in the brain. GSMs, however, have no effect, even at very high concentrations, on Notch proteolysis and signaling, nor do they elevate γ-secretase substrates. Presumably for these reasons, these compounds have shown excellent safety profiles, both in animal models and in human trials.
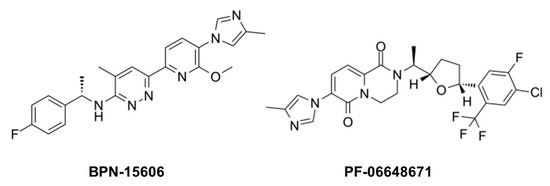
Figure 210. Example γ-secretase modulators (GSMs).
The mechanism of action of these compounds is not entirely clear, although the correlation between Aβ42 lowering and Aβ38 elevation is relevant, as Aβ42 is a precursor to Aβ38. γ-Secretase cleaves the Aβ42 C-terminus to release a tetrapeptide [23][45], and isolated γ-secretase converts synthetic Aβ42 to Aβ38 with release of this tetrapeptide [24][87]. Moreover, presenilin mutations decrease the Aβ42-to-Aβ38 conversion while GSMs stimulate it. Thus, GSMs appear to decrease Aβ42 by enhancing the carboxypeptidase activity of γ-secretase that converts this aggregation-prone peptide to Aβ38.
A critical issue with GSMs, however, like all anti-Aβ therapeutic strategies, is the design of clinical trials [25][88]. So far, all reported clinical trials with candidate AD therapeutic agents, including GSIs, GSMs and anti-Aβ immunotherapy, have been with individuals who already have AD or a pre-AD condition called mild cognitive impairment (MCI). Even in those with MCI, substantial neurodegeneration has occurred, and there are serious concerns that targeting Aβ after the onset of symptoms is too late. Aβ pathology in the brain may appear more than 10 years before the clinical manifestation of AD [26][89]. As Aβ pathology apparently precedes tau pathology [27][28][4,90], and tau pathology may then propagate from neuron to neuron [29][30][91,92], blocking Aβ after tau pathology is initiated may not prevent or slow the progression of AD and may not even prevent or delay disease onset. For anti-Aβ strategies—including GSMs—to succeed, clearer knowledge of the pathogenic process and timing is needed, as are convenient and reliable biomarkers and diagnostics.
Moreover, the clinical success of GSMs is completely dependent on whether Aβ42 is indeed the pathogenic entity in AD. The reasons for the focus on Aβ42 are arguably more historic than a result of an objective search with no preconceptions. Over 100 years ago, Alois Alzheimer described extraneuronal amyloid plaques as a signature pathological characteristic of the disease, and the discovery in the late 1980s and early 1990s that the primary protein component of the plaques is Aβ [31][32][93,94], with Aβ42 being the predominant species [33][13] and most aggregation-prone [34][95], led to the assumption that Aβ42 is the likely pathogenic species. Subsequent studies ranging from effects of APP and presenilin mutations to the neurotoxicity of various Aβ assemblies would seem to confirm this hypothesis. However, selectivity in the reporting of findings (negative results are more difficult to publish) in combination with incomplete knowledge of all forms of Aβ could result in mistaking correlation for causality. In light of more recent findings, mentioned earlier, that FAD mutations in PSEN1 increase Aβ peptide intermediates of 45 residues and longer, it would seem worthwhile to determine the effects of GSMs on the first or second trimming events of APP substrate by γ-secretase (e.g., Aβ49→46, Aβ45→42).
