Among oxide semiconductors,
p
-type Mn
3
O
4
systems have been exploited in chemo-resistive sensors for various analytes, but their use in the detection of H
2
, an important, though flammable, energy vector, has been scarcely investigated. Herein, we report for the first time on the plasma assisted-chemical vapor deposition (PA-CVD) of Mn
3
O
4
nanomaterials, and on their on-top functionalization with Ag and SnO
2
by radio frequency (RF)-sputtering, followed by air annealing. The obtained Mn
3
O
4
-Ag and Mn
3
O
4
-SnO
2
nanocomposites were characterized by the occurrence of phase-pure tetragonal α-Mn
3
O
4
(hausmannite) and a controlled Ag and SnO
2
dispersion. The system functional properties were tested towards H
2
sensing, yielding detection limits of 18 and 11 ppm for Mn
3
O
4
-Ag and Mn
3
O
4
-SnO
2
specimens, three orders of magnitude lower than the H
2
explosion threshold. These performances were accompanied by responses up to 25% to 500 ppm H
2
at 200 °C, superior to bare Mn
3
O
4
, and good selectivity against CH
4
and CO
2
as potential interferents. A rationale for the observed behavior, based upon the concurrence of built-in Schottky (Mn
3
O
4
/Ag) and
p
-
n
junctions (Mn
3
O
4
/SnO
2
), and of a direct chemical interplay between the system components, is proposed to discuss the observed activity enhancement, which paves the way to the development of gas monitoring equipments for safety end-uses.
- Mn3O4
- Ag
- SnO2
- plasma assisted-chemical vapor deposition
- hydrogen gas sensors
1. Introduction
The reliable detection of hazardous/flammable gases is of key importance in a variety of fields, encompassing disease diagnosis, environmental monitoring and human health protection [1][2][3][4][5][6]. In this broad scenario, a key role is played by the early recognition of molecular hydrogen (H
The reliable detection of hazardous/flammable gases is of key importance in a variety of fields, encompassing disease diagnosis, environmental monitoring and human health protection [1,2,3,4,5,6]. In this broad scenario, a key role is played by the early recognition of molecular hydrogen (H
2
), a zero-emission and clean fuel, with a high energy density of ≈130 MJ×kg
−1 [2][7], which has emerged as a future energy source for transportation, industrial and residential applications [8][9][10]. Nevertheless, since H
[2,7], which has emerged as a future energy source for transportation, industrial and residential applications [8,9,10]. Nevertheless, since H
2 is colorless, odorless and highly flammable, the detection of hydrogen leakages at concentrations lower than hazardous levels [11][12][13][14] is extremely critical towards the emergence of a future hydrogen economy [7][8][15][16][17][18][19].
is colorless, odorless and highly flammable, the detection of hydrogen leakages at concentrations lower than hazardous levels [11,12,13,14] is extremely critical towards the emergence of a future hydrogen economy [7,8,15,16,17,18,19].
Simple architecture, cost-effective fabrication, stability under the operating conditions, and high efficiency, are the main requirements and core features of advanced sensors needed for such applications [16]. Among the various active systems and devices [20][21][22][23][24], metal oxide nanostructures have been the subject of an increasing interest, thanks to their high carrier mobility, easy fabrication and excellent stability [9][25][26][27][28]. In particular, whereas
Simple architecture, cost-effective fabrication, stability under the operating conditions, and high efficiency, are the main requirements and core features of advanced sensors needed for such applications [16]. Among the various active systems and devices [20,21,22,23,24], metal oxide nanostructures have been the subject of an increasing interest, thanks to their high carrier mobility, easy fabrication and excellent stability [9,25,26,27,28]. In particular, whereas
n-type oxide semiconductors have been largely investigated as gas sensors [8][9][12][15],
-type oxide semiconductors have been largely investigated as gas sensors [8,9,12,15],
p-type ones have not yet been widely studied [4][17][29][30], since their responses are typically lower than those of
-type ones have not yet been widely studied [4,17,29,30], since their responses are typically lower than those of
n-type systems with comparable morphology [31][32][33]. Nonetheless,
-type systems with comparable morphology [31,32,33]. Nonetheless,
p-type oxide semiconductors have an important potential as gas sensors, and represent promising platforms for the development of devices exhibiting new functions [32], taking into account their appreciable activity as oxidation catalysts and the possibility of boosting their performances by tailoring their chemico-physical properties [2][31][34][35].
-type oxide semiconductors have an important potential as gas sensors, and represent promising platforms for the development of devices exhibiting new functions [32], taking into account their appreciable activity as oxidation catalysts and the possibility of boosting their performances by tailoring their chemico-physical properties [2,31,34,35].
Among
p
-type systems, Mn
3
O
4 has received significant attention due to its low cost, large natural abundance, environmentally friendly character and versatile chemico-physical properties, including the coexistence of mixed valence states [36][37]. Over the last decade, different studies have reported on Mn
has received significant attention due to its low cost, large natural abundance, environmentally friendly character and versatile chemico-physical properties, including the coexistence of mixed valence states [36,37]. Over the last decade, different studies have reported on Mn
3
O
4
-containing gas sensors for various analytes, including CH
3
CH
2
OH, CH
3
COCH
3
, NH
3 and chemical warfare agent simulants [31][35][36][37][38][39][40][41]. Nonetheless, only two works on Mn
and chemical warfare agent simulants [31,35,36,37,38,39,40,41]. Nonetheless, only two works on Mn
3
O
4-based gas sensors for molecular hydrogen detection are available in the literature so far [35][42], and the implementation of H
-based gas sensors for molecular hydrogen detection are available in the literature so far [35,42], and the implementation of H
2 sensors endowed with improved sensitivity and selectivity undoubtedly requires additional research efforts [29][35][37][38].
sensors endowed with improved sensitivity and selectivity undoubtedly requires additional research efforts [29,35,37,38].
Beside tailoring the system morphology [25][26][33][39], a proficient way to enhance the functionality of bare Mn
Beside tailoring the system morphology [25,26,33,39], a proficient way to enhance the functionality of bare Mn
3
O
4 gas sensors involves their sensitization with suitable metal/oxide agents [8][18][35][42][43][44]. The ultimate aim of this strategy is the exploitation of synergistical chemical and electronic effects, in order to obtain improved performances at moderate working temperatures, an issue of key importance for the development of low power consumption devices [4][37][41]. In this context, the present study is devoted to the fabrication of Mn
gas sensors involves their sensitization with suitable metal/oxide agents [8,18,35,42,43,44]. The ultimate aim of this strategy is the exploitation of synergistical chemical and electronic effects, in order to obtain improved performances at moderate working temperatures, an issue of key importance for the development of low power consumption devices [4,37,41]. In this context, the present study is devoted to the fabrication of Mn
3
O
4
-based chemo-resistive sensors for H
2 detection, sensitized through the on-top deposition of selected metal and metal oxide activators. As prototypes for the two categories, in this work our attention has been focused on the use of Ag, a potential catalyst promoting the reactions involved in the sensing process [45][46][47][48][49], and of SnO
detection, sensitized through the on-top deposition of selected metal and metal oxide activators. As prototypes for the two categories, in this work our attention has been focused on the use of Ag, a potential catalyst promoting the reactions involved in the sensing process [45,46,47,48,49], and of SnO
2, by far one of the most investigated metal oxides for gas sensing applications, endowed with high electron mobility and gas sensitivity [10][13][25][26][50]. In particular, the occurrence of Schottky (Mn
, by far one of the most investigated metal oxides for gas sensing applications, endowed with high electron mobility and gas sensitivity [10,13,25,26,50]. In particular, the occurrence of Schottky (Mn
3
O
4
/Ag) or
p
-
n
(Mn
3
O
4
/SnO
2) junctions between the system components can indeed enhance the modulations of the space charge region, and of the measured electrical resistance, ultimately yielding improved sensing performances thanks to electronic effects [18][26][33][43].
) junctions between the system components can indeed enhance the modulations of the space charge region, and of the measured electrical resistance, ultimately yielding improved sensing performances thanks to electronic effects [18,26,33,43].
At variance with our previous studies, which have involved the fabrication of Mn
3
O
4-based sensors by means of thermally activated chemical vapor deposition (CVD)-based processes [31][38][39][41], in this work a novel two-step plasma-assisted route was adopted for the preparation of the present materials. The fabrication procedure (
-based sensors by means of thermally activated chemical vapor deposition (CVD)-based processes [31,38,39,41], in this work a novel two-step plasma-assisted route was adopted for the preparation of the present materials. The fabrication procedure (
) involved: (i) the initial plasma assisted-CVD (PA-CVD) on alumina substrates of MnO
2
from Mn(hfa)
2
TMEDA (Hhfa = 1,1,1,5,5,5-hexafluoro-2,4-pentanedione; TMEDA =
N
,
N
,
N’
,
N’-tetramethylethylenediamine) [51][52], a molecular precursor never utilized so far for PA-CVD processes; (ii) the functionalization with Ag or SnO
-tetramethylethylenediamine) [51,52], a molecular precursor never utilized so far for PA-CVD processes; (ii) the functionalization with Ag or SnO
2
by means of radio frequency (RF)-sputtering, and (iii) a final thermal treatment in air to trigger the transformation of MnO
2
into Mn
3
O
4
. The main focus of the present investigation was directed at elucidating the structural, compositional and morphological characteristics of the target materials and their interplay with the resulting sensing performances in hydrogen detection. The latter were investigated at a fixed humidity level as a function of the operating temperature, with particular regard to the role exerted by the formation of metal-oxide (Mn
3
O
4
/Ag) or oxide-oxide (Mn
3
O
4
/SnO
2
) junctions. The obtained results indicate that the proposed preparation method yields H
2
sensors exhibiting favorable detection limits, promising responses at moderate temperature, as well as selectivity against carbon dioxide and methane as potential interferents. To the best of our knowledge, this is the first report on hydrogen gas sensing by Mn
3
O
4
-based composites prepared by a plasma-assisted route.
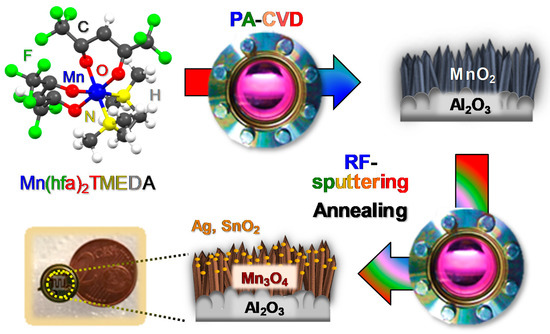
Figure 1.
3
4
3
4
2
2. Results and Discussion
2.1. Chemico-Physical Characterization
The fabrication process of the target materials is illustrated in
. Particular efforts were dedicated to elucidating the interplay between the adopted processing conditions and material chemical, physical and functional properties.
The system structure was investigated by XRD analyses (
). All the observed reflections located at 2
θ
= 18.0°, 28.9°, 31.0°, 32.3° and 36.1° could be indexed to the (101), (112), (200), (103) and (211) planes of tetragonal hausmannite (α-Mn
3
O
4
;
a
= 5.762 Å and
c = 9.470 Å [31][38][53]). The occurrence of relatively weak and broad diffraction peaks suggested the formation of defective nanocrystallites [11], whose average dimensions were close to 25 nm for all the target specimens. A comparison of the signal relative intensities with those of the reference pattern [53] did not reveal any significant orientation/texturing effect, and no appreciable reflections from other Mn oxide polymorphs could be distinguished, highlighting the occurrence of phase-pure systems. Upon functionalization of Mn
= 9.470 Å [31,38,59]). The occurrence of relatively weak and broad diffraction peaks suggested the formation of defective nanocrystallites [11], whose average dimensions were close to 25 nm for all the target specimens. A comparison of the signal relative intensities with those of the reference pattern [59] did not reveal any significant orientation/texturing effect, and no appreciable reflections from other Mn oxide polymorphs could be distinguished, highlighting the occurrence of phase-pure systems. Upon functionalization of Mn
3
O
4
by RF-sputtering, no net variation in the recorded XRD patterns took place. The absence of noticeable diffraction peaks related to Ag or SnO
2
was traced back to their low content and high dispersion into the Mn
3
O
4 systems [19][41][44][46] (see also XPS and SIMS results).
systems [19,41,44,46] (see also XPS and SIMS results).
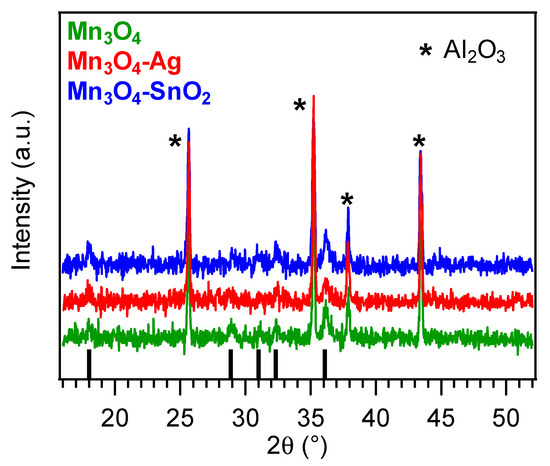
Figure 2. X-ray diffraction (XRD patterns for bare and functionalized Mn3O4 nanosystems. Vertical black bars correspond to α-Mn3O4 signals [53][59].
The surface chemical state of the developed materials was analyzed by means of XPS.
a displays the survey spectra for the target specimens, that revealed the presence of oxygen, manganese and eventually, silver or tin signals, for the functionalized systems, together with a minor carbon contribution (<10 at.%) resulting from adventitious contamination. The detection of manganese signals even after RF-sputtering suggested only a partial coverage of Mn
3
O
4
by the deposited silver- and tin-containing species. Accordingly, Ag and Sn molar fractions were evaluated to be 47.0% and 31.0%, respectively. For bare Mn
3
O
4
, the Mn2p
3/2
component was located at BE = 641.8 eV (spin-orbit splitting (SOS) = 11.5 eV,
Figure 3b), in accordance with previous literature data [31][35][38][41]. For the functionalized systems, a lower Mn2p
b), in accordance with previous literature data [31,35,38,41]. For the functionalized systems, a lower Mn2p
3/2
BE was observed (641.7 eV, for Mn
3
O
4
-Ag, and 641.5 eV, for Mn
3
O
4
-SnO
2
). This finding suggested the formation of Schottky and
p
-
n
junctions for Mn
3
O
4
-Ag and Mn
3
O
4
-SnO
2, respectively [33][37][38][41][42], resulting in an Ag → Mn
, respectively [33,37,38,41,42], resulting in an Ag → Mn
3
O
4
and SnO
2
→ Mn
3
O
4
electron transfer. This phenomenon, more pronounced for SnO
2
-containing samples, as testified by the higher BE decrease, exerted a favorable influence on the resulting gas sensing performances. As regards silver (
c), the Ag3d
5/2
position (BE = 368.5 eV, SOS = 6.0 eV), as well as the pertaining Auger parameters (see also
;
α1
= 719.7 eV and
α2 = 725.6 eV), revealed a partial Ag surface oxidation, i.e., the coexistence of Ag(0) and Ag(I) oxide, as typically observed in similar cases [38][46][49][54]. Finally, the main tin photopeak (
= 725.6 eV), revealed a partial Ag surface oxidation, i.e., the coexistence of Ag(0) and Ag(I) oxide, as typically observed in similar cases [38,46,49,60]. Finally, the main tin photopeak (
d; BE(Sn3d
5/2
) = 486.9 eV; SOS = 8.4 eV) was located at higher energies than those reported for SnO
2 [14][55][56], in line with the above mentioned charge transfer process. Taken together, these results highlighted the formation of nanocomposites in which the single components maintained their chemical identity, and enabled us to discard the formation of ternary phases, in line with XRD results. The deconvolution of O1s photopeaks (
[14,56,61], in line with the above mentioned charge transfer process. Taken together, these results highlighted the formation of nanocomposites in which the single components maintained their chemical identity, and enabled us to discard the formation of ternary phases, in line with XRD results. The deconvolution of O1s photopeaks (
) revealed the concurrence of two distinct bands at BE = 530.0 eV, resulting from lattice oxygen in Mn
3
O
4
, Ag(I) oxide (Mn
3
O
4
-Ag) or SnO
2
(Mn
3
O
4
-SnO
2) [14][31][38][54][56], and 531.6 eV, assigned to oxygen species adsorbed on surface O defects [4][41][51][52][57]. The contribution of the latter component to the overall O1s signal increased from ≈36.0%, for bare Mn
) [14,31,38,60,61], and 531.6 eV, assigned to oxygen species adsorbed on surface O defects [4,41,51,52,55]. The contribution of the latter component to the overall O1s signal increased from ≈36.0%, for bare Mn
3
O
4
, to ≈58.0%, for the functionalized specimens, indicating a parallel increase of the oxygen defect content. The latter feature had a direct beneficial impact on the resulting gas sensing behavior.
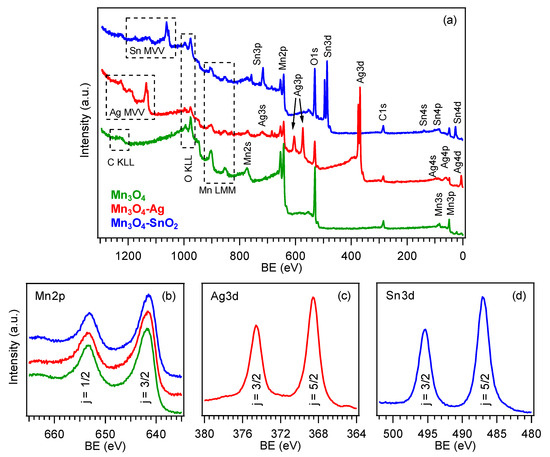
Figure 3. (a) X-ray photoelectron spectroscopy (XPS) wide-scan spectra pertaining to bare Mn3O4, Mn3O4-Ag and Mn3O4-SnO2 samples. (b) Mn2p, (c) Ag3d and (d) Sn3d photoelectron peaks. The color code is reported in panel (a).
Complementary information on material chemical composition was obtained by SIMS in-depth profiling (
). Upon functionalization of Mn
3
O
4
, no significant variations in the overall deposit thickness took place (for all specimens, the average value was (400 ± 50) nm, as determined by cross-sectional FE-SEM analyses (see below and
)). The almost parallel trends of manganese and oxygen ionic yields suggested their common chemical origin, in line with the formation of phase-pure Mn
3
O
4
. Silver and tin trends could be described by an erfchian profile, such as in thermal diffusion processes [49]. For the Mn
3
O
4
-SnO
2
sample, Sn yield underwent a progressive decrease throughout the outer 100 nm, subsequently followed by a plateau, whereas, for the Mn
3
O
4
-Ag specimen, the silver curve continuously declined even at higher depth values. In spite of these differences, a penetration of both Ag and Sn up to the interface with the alumina substrate was observed, and ascribed to the synergistical combination between the inherent RF-sputtering infiltration power and the Mn
3
O
4 deposit open morphology [38][41][44][48] (see also
deposit open morphology [38,41,44,48] (see also
). This intimate contact between the system components is indeed an issue of key importance in order to benefit from their mutual electronic interplay, as discussed in detail below.
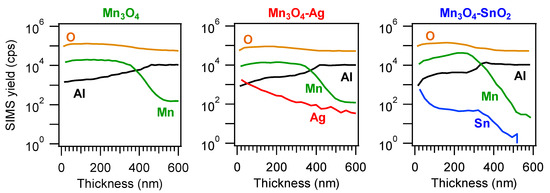
Figure 4.
3
4
3
4
3
4
2
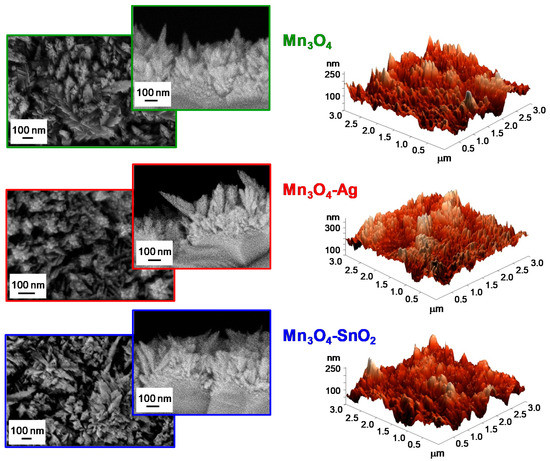
Figure 5. Representative plane-view and cross-sectional field emission-scanning electron microscopy (FE-SEM) micrographs (left panels) and atomic force microscopy (AFM) images (right panels) for Mn3O4, Mn3O4-Ag and Mn3O4-SnO2 samples.
The system morphology was investigated by the complementary use of FE-SEM and AFM. FE-SEM micrographs (see
, left side) highlighted that bare Mn
3
O
4 was characterized by the presence of elongated nanoaggregates (mean size = 100 nm), whose interconnection resulted in the formation of arrays with an open morphology. This feature is indeed favorable in view of gas sensing applications, since a higher area available for the interaction with the surrounding gases has a beneficial effect on the ultimate material functional performances [15][19][25][31][40]. After Ag and SnO
was characterized by the presence of elongated nanoaggregates (mean size = 100 nm), whose interconnection resulted in the formation of arrays with an open morphology. This feature is indeed favorable in view of gas sensing applications, since a higher area available for the interaction with the surrounding gases has a beneficial effect on the ultimate material functional performances [15,19,25,31,40]. After Ag and SnO
2
introduction, no marked variations involving aggregate coalescence/collapse could be observed, validating the potential of the adopted synthetic route in functionalizing Mn
3
O
4
nano-deposits without any undesired morphological alteration. AFM analyses (
, right side) confirmed the presence of the aforementioned aggregates uniformly protruding from the growth substrate, resulting in a crack-free and homogeneous granular topography, yielding an average RMS surface roughness of 40 nm for all the analyzed specimens.
2.2. Gas Sensing Performances
displays representative dynamical responses of the developed sensors towards square concentration pulses of gaseous hydrogen. All the target materials exhibited a
p
-type sensing behavior, as indicated by the resistance increase upon H
2
exposure due to the reaction of the analyte with adsorbed oxygen species, resulting in a decrease of the major
p-type carrier concentration [2][16][31][35][39]. This phenomenon is in agreement with the fact that Mn
-type carrier concentration [2,16,31,35,39]. This phenomenon is in agreement with the fact that Mn
3
O
4 is the main system component, as indicated by structural and compositional characterization [44].
is the main system component, as indicated by structural and compositional characterization [44].
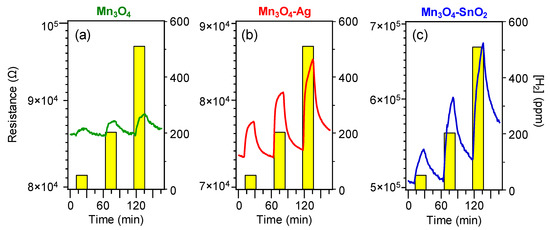
Figure 6. Dynamical responses of Mn3O4 (a), Mn3O4-Ag (b) and Mn3O4-SnO2 (c) nanosystems vs. different H2 concentrations, at a fixed working temperature of 200 °C.
Remarkably, data in
evidence that the on-top deposition of Ag and SnO
2
was an effective mean to increase the electrical property modulation upon H
2
exposure with respect to pure Mn
3
O
4. For both composite systems, the measured resistance underwent a relatively sharp rise upon hydrogen exposure, and a subsequent slower increase up to the end of each gas pulse. This phenomenon suggested that the rate-limiting step in the resistance change was the chemisorption of molecular hydrogen on the sensor surface [15][31][41][48]. In spite of an incomplete baseline recovery after switching off hydrogen pulses, the measured resistance variations were almost proportional to the used hydrogen concentrations, enabling us to rule out significant saturation effects, an important starting point for eventual practical applications [15][38][39].
. For both composite systems, the measured resistance underwent a relatively sharp rise upon hydrogen exposure, and a subsequent slower increase up to the end of each gas pulse. This phenomenon suggested that the rate-limiting step in the resistance change was the chemisorption of molecular hydrogen on the sensor surface [15,31,41,48]. In spite of an incomplete baseline recovery after switching off hydrogen pulses, the measured resistance variations were almost proportional to the used hydrogen concentrations, enabling us to rule out significant saturation effects, an important starting point for eventual practical applications [15,38,39].
To account for the performance increase yielded by composite systems, it is necessary to consider the mechanism of hydrogen detection by the target
p-type materials, which can be described as follows. Upon air exposure prior to contact with the target analyte, oxygen molecules undergo chemisorption processes, yielding the formation of various species [6][9][29][36][43][57], among which O
-type materials, which can be described as follows. Upon air exposure prior to contact with the target analyte, oxygen molecules undergo chemisorption processes, yielding the formation of various species [6,9,29,36,43,55], among which O
− is the prevailing one in the present working temperature interval [14][17][30][33]:
is the prevailing one in the present working temperature interval [14,17,30,33]:
2 (g)
−
(ads)
h+
As a consequence, the formation of a low resistance hole accumulation layer (HAL) in the near surface Mn
3
O
4
region takes place (
; HAL thickness = 20.6 nm, see the
Supporting Information) [32][33][37][47]. The subsequent analyte chemisorption is accompanied by electron injection into the system conduction band [3][4][7][29][40][44][58]:
) [32,33,37,47]. The subsequent analyte chemisorption is accompanied by electron injection into the system conduction band [3,4,7,29,40,44,62]:
H
2 (ads)
+ O
−
(ads)
⇄ H
2
O
(g)
+
e−
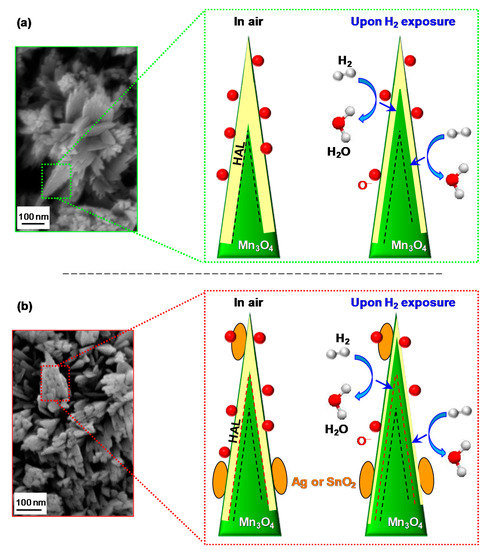
Figure 7.
Schematics of the hydrogen gas sensing mechanism and corresponding hole accumulation layer (HAL) modulation for nanosystems based on: (
a
) bare Mn
3
O
4
; (
b
) functionalized Mn
3
O
4
. The dashed black and red lines indicate the HAL boundaries in air in case of bare and functionalized Mn
3
O
4
, respectively. Red spheres, yellow areas, and orange ovals indicate adsorbed oxygen, HAL thickness, and functionalizing agent (Ag or SnO
2
), respectively. Blue arrows indicate electron flow due to H
2
oxidation (see reaction 4).
A process which decreases the hole concentration and the HAL thickness, resulting, in turn, in an increase of the measured resistance [5][16][17][18][42][43]. Finally, upon switching off the gas pulse, the sensor surface is again in contact with air, and the original situation is restored, with a recovery of the pristine HAL width [30][31][41].
A process which decreases the hole concentration and the HAL thickness, resulting, in turn, in an increase of the measured resistance [5,16,17,18,42,43]. Finally, upon switching off the gas pulse, the sensor surface is again in contact with air, and the original situation is restored, with a recovery of the pristine HAL width [30,31,41].
Since all the investigated systems have almost identical mean crystallite dimensions, grain size and RMS roughness values, a significant influence of these parameters on the different gas sensing performances can be reasonably ruled out. Indeed, the enhanced responses of composite sensors with respect to the pristine Mn
3
O
4
can be first explained in terms of electronic effects occurring at the interface between Mn
3
O
4 and the functionalizing agents, a key aspect to be considered for a deep understanding of gas sensing phenomena [26].
and the functionalizing agents, a key aspect to be considered for a deep understanding of gas sensing phenomena [26].
For Mn
3
O
4
-Ag sensors, these processes result from the formation of Mn
3
O
4
/Ag Schottky junctions, whose occurrence produces a Ag → Mn
3
O
4 electron flow [38][59] (see also the above XPS data), and a consequent thinning of the HAL width in comparison with bare Mn
electron flow [38,63] (see also the above XPS data), and a consequent thinning of the HAL width in comparison with bare Mn
3
O
4
(compare
a,b). As a consequence, HAL variations upon contact of the sensor with gaseous H
2 produce higher responses by increasing the registered resistance modulations [38][44]. An analogous phenomenon occurs for Mn
produce higher responses by increasing the registered resistance modulations [38,44]. An analogous phenomenon occurs for Mn
3
O
4
-SnO
2
systems (
b; HAL thickness = 12.4 nm, see the
), although in this case the SnO
2
→ Mn
3
O
4
electron flow is triggered by a different phenomenon, i.e., the presence of
p
-
n
Mn
3
O
4
/SnO
2 junctions [32][33][37][41][42][43][45].
junctions [32,33,37,41,42,43,55].
The latter effect can, in principle, result in enhanced variations of the HAL extension with respect to the case of Mn
3
O
4
-Ag sensors, since the occurrence of a partial silver oxidation (as evidenced by XPS analysis, see above) precludes a full exploitation of electron transfer effects resulting from the establishment of Mn
3
O
4/Ag Schottky junctions [38][46].
/Ag Schottky junctions [38,46].
Nonetheless, the enhanced hydrogen detection efficiency of Mn
3
O
4
-based nanocomposites with respect to bare manganese oxide is likely due to the concurrence of additional cooperative phenomena. For both Mn
3
O
4
-Ag and Mn
3
O
4
-SnO
2
systems, the higher content of oxygen defects at the composite surface with respect to bare Mn
3
O
4
(see the above XPS data and
Figure S2), as well as the exposure of a high density of heterointerfaces, can in fact supply active sites for a more efficient chemisorption of both oxygen and analyte molecules, which, in turn, boosts the resulting gas responses [4][8][16][17][18][30][43]. In addition, the intimate component contact enabled by the adopted preparation route, yielding a good intergranular coupling, enables a proficient exploitation of their chemical interplay [38][44][48], related to the synergistical combination of materials with different catalytic activities [10][27][37][41][47]. Hence, the improved sensing performances of functionalized Mn
), as well as the exposure of a high density of heterointerfaces, can in fact supply active sites for a more efficient chemisorption of both oxygen and analyte molecules, which, in turn, boosts the resulting gas responses [4,8,16,17,18,30,43]. In addition, the intimate component contact enabled by the adopted preparation route, yielding a good intergranular coupling, enables a proficient exploitation of their chemical interplay [38,44,48], related to the synergistical combination of materials with different catalytic activities [10,27,37,41,47]. Hence, the improved sensing performances of functionalized Mn
3
O
4
systems can be related to the concomitance of electronic and catalytic effects.
Taken together, the above observations can account for the improved performances at lower working temperatures of SnO
2
-containing systems with respect to Ag-containing ones. This result is exemplified by an inspection of
, showing that, apart from the appreciable response enhancement, deposition of Ag and SnO
2
onto Mn
3
O
4
resulted in different trends as a function of the operating temperature. In the case of Mn
3
O
4-Ag sensors, the progressive response rise indicated an enhanced extent of reaction (4) upon increasing the thermal energy supply [2][8][15][31][38][39]. Conversely, as concerns Mn
-Ag sensors, the progressive response rise indicated an enhanced extent of reaction (4) upon increasing the thermal energy supply [2,8,15,31,38,39]. Conversely, as concerns Mn
3
O
4
-SnO
2
, a maximum-like response behavior was observed, the best operating temperature being 200 °C. Such a response trend, in line with previous reports regarding H
2 detection by other metal oxides [7][9][17][19][29][43][60], suggested the occurrence of a steady equilibrium between hydrogen adsorption and desorption at 200 °C, whereas an increase of the working temperature resulted in a predominant analyte desorption [27][33][57][60][61]. The lower value of the optimum operating temperature for Mn
detection by other metal oxides [7,9,17,19,29,43,64], suggested the occurrence of a steady equilibrium between hydrogen adsorption and desorption at 200 °C, whereas an increase of the working temperature resulted in a predominant analyte desorption [27,33,55,64,65]. The lower value of the optimum operating temperature for Mn
3
O
4
-SnO
2
in comparison to Mn
3
O
4
-Ag is in line with the more efficient SnO
2
→ Mn
3
O
4
electron transfer (see the above XPS data).
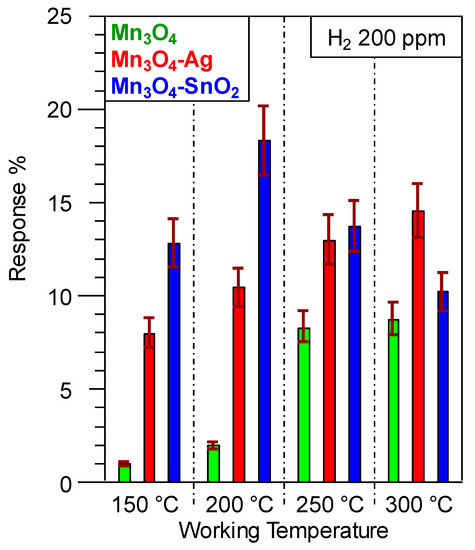
Figure 8.
2
3
4
The potential of the present results is highlighted by the fact that the best H
2
responses obtained for Mn
3
O
4
-Ag (at 300 °C) and Mn
3
O
4
-SnO
2
(at 200 °C) were higher than those reported for sensors based not only on Mn
3
O
p-type oxides, including CuO [3][29], NiO [2][30], BiFeO
-type oxides, including CuO [3,29], NiO [2,30], BiFeO
3 [7], Co
[7], Co
3
O
x
Co
3-x
O
4 [16], as well as nanocomposites based on CuO-Pt [4] and CuO-WO
[16], as well as nanocomposites based on CuO-Pt [4] and CuO-WO
3 [18]. In addition, the same responses compared favorably with those pertaining to various MnO
[18]. In addition, the same responses compared favorably with those pertaining to various MnO
2-based nanomaterials/thin films in the detection of the same analyte [1][60][61]. A comparison of selected representative data is reported in
-based nanomaterials/thin films in the detection of the same analyte [1,64,65]. A comparison of selected representative data is reported in
. It is also worthwhile highlighting that the optimal operating temperature for H
2
detection by the present materials (200 °C for Mn
3
O
4
-SnO
2
systems) was lower than the ones reported for Mn
3
O
4 [35], MnO
[35], MnO
3
O
4 [16], NiO [9], NiO-ZnO [43], Ni
[16], NiO [9], NiO-ZnO [43], Ni
x
Co
3-x
O
4 [16], BiFeO
[16], BiFeO
3 [7] and CuO-WO
[7] and CuO-WO
3 sensors [18]. This result is of importance, not only to avoid dangerous temperature-triggered explosions, but also to implement sensing devices with a higher service life and a lower power consumption [11][25][38][41].
sensors [18]. This result is of importance, not only to avoid dangerous temperature-triggered explosions, but also to implement sensing devices with a higher service life and a lower power consumption [11,25,38,41].
Gas responses were also analyzed as a function of H
2
concentration (
Figure S3). The obtained linear trends in the log-log scale confirmed the absence of appreciable saturation phenomena, an important prerequisite for a quantitative analyte detection [37][38][39][44][48]. The best detection limits obtained by fitting experimental data with equation (2) ((18 ± 1) ppm and (11 ± 1) ppm for Mn
). The obtained linear trends in the log-log scale confirmed the absence of appreciable saturation phenomena, an important prerequisite for a quantitative analyte detection [37,38,39,44,48]. The best detection limits obtained by fitting experimental data with equation (2) ((18 ± 1) ppm and (11 ± 1) ppm for Mn
3
O
4
-Ag and Mn
3
O
4
-SnO
2
sensors, respectively) were close to those previously reported for MnO
2
-Au [44] sensors, and inferior than those pertaining to ZnO ones [15]. It is also worth noticing that these values were nearly three orders of magnitude lower than the H
2 lower explosion limit (LEL, 40000 ppm) [2][11][12][23][43], highlighting thus the detection efficiency of the present systems.
lower explosion limit (LEL, 40000 ppm) [2,11,12,23,43], highlighting thus the detection efficiency of the present systems.
Beyond sensitivity, selectivity is an important parameter for the eventual utilization of gas sensing devices [6][11][19][27][30]. The responses towards a specific test gas are in fact required to be higher than those of other potential interferents, in order to avoid false alarms in real-time gas monitoring equipment [36][38][39]. In particular, the choice of CH
Beyond sensitivity, selectivity is an important parameter for the eventual utilization of gas sensing devices [6,11,19,27,30]. The responses towards a specific test gas are in fact required to be higher than those of other potential interferents, in order to avoid false alarms in real-time gas monitoring equipment [36,38,39]. In particular, the choice of CH
4
and CO
2 as potential interferents in real-time hydrogen leak detection is motivated by the fact that: (i) hydrogen and methane are common reducing gases, either stored, or used together [1][13]; (ii) the presence of carbon dioxide may hamper hydrogen recognition [5][21][43] in the case of fuel cells eliminating CO
as potential interferents in real-time hydrogen leak detection is motivated by the fact that: (i) hydrogen and methane are common reducing gases, either stored, or used together [1,13]; (ii) the presence of carbon dioxide may hamper hydrogen recognition [5,21,43] in the case of fuel cells eliminating CO
2
and producing electricity and H
2 [62]. As shown in
[66]. As shown in
, the present sensors yielded no responses towards CO
2
, and only weak signals upon exposure to CH
4
. In the latter case, the selectivity was estimated as the ratio between the responses to H
2
and CH
4 [32][41], yielding values of 22 and 24 for Mn
[32,41], yielding values of 22 and 24 for Mn
3
O
4
-Ag and Mn
3
O
4
-SnO
2
at the best working temperatures (300 and 200 °C, respectively). Though preliminary in nature, the latter results are an attractive starting point for further studies aimed at implementing exclusive H
2 sensors, which are highly required for practical applications [13].
sensors, which are highly required for practical applications [13].
