Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases that are responsible for the degradation of a wide range of extracellular matrix proteins, which are involved in many cellular processes to ensure the normal development of tissues and organs. Overexpression of MMPs has been observed to facilitate cellular growth, migration, and metastasis of tumor cells during cancer progression. A growing number of these proteins are being found to exist in the nuclei of both healthy and tumor cells, thus highlighting their localization as having a genuine purpose in cellular homeostasis. The mechanism underlying nuclear transport and the effects of MMP nuclear translocation have not yet been fully elucidated. To date, nuclear MMPs appear to have a unique impact on cellular apoptosis and gene regulation, which can have effects on immune response and tumor progression, and thus present themselves as potential therapeutic targets in certain types of cancer or disease. Herein, we highlight and evaluate what progress has been made in this area of research, which clearly has some value as a specific and unique way of targeting the activity of nuclear matrix metalloproteinases within various cell types.
- Nuclear Matrix Metalloproteinases,Matrix Metalloproteinases,cancer
1. Introduction
Matrix metalloproteinases (MMPs) are involved in the degradation of extracellular matrix (ECM) proteins and regulate many fundamental cellular processes during normal bodily development and function [1]. As the ECM is important in maintaining the mechanical and biochemical properties of tissues, its normal turnover and regulation by MMPs is necessary to permit multiple functions, as in the cleavage and activation of signaling molecules, cellular differentiation, and wound healing [2][3][4][5][6][7]. However, dysregulation of MMP activity can contribute to a variety of pathological conditions. For example, some have been seen to modulate matrix erosion in osteoarthritis and rheumatoid arthritis, whereas expression of others is associated with the formation of atherosclerotic lesions, platelet aggregation, and the regulation of factors associated with cardiovascular disease [8][9]. Predominantly, the roles of MMPs in malignant tumor initiation, metastasis, and angiogenesis have received the greatest attention and which have highlighted them as good potential therapeutic targets for the treatment of certain types of cancer [9].
To date, 26 human MMP proteins have been identified, which belong to the M10 family of metallo-endopeptidases [10]. Based on substrate specificity, MMPs can be further categorized into collagenases (MMP-1, MMP-8, MMP-13, and MMP-18), gelatinases (MMP-2 and MMP-9), stromelysins (MMP-3, MMP-10, MMP-11, and MMP-17), matrilysins (MMP-7 and MMP-26), membrane-type MMPs (MMP-14, MMP-15, MMP-16, MMP-17, MMP-24, and MMP-25), and others (MMP-12, MMP-19, MMP-20, MMP-21, MMP-22, MMP-23, MMP-28, and MMP-29) [1]. Generally speaking, they are expressed by a broad range of cell types, such as epithelial cells, fibroblasts, osteoblasts, endothelial cells, vascular smooth muscle, macrophages, neutrophils, lymphocytes, and cytotrophoblasts [1].
Structurally, MMPs share a common protein domain structure (Figure 1). For most MMPs, the main components are a signal peptide (that directs synthesized protein into the secretory pathway), a highly conserved amino-terminal pro-domain, a catalytic domain that contains a zinc ion binding site, a linker domain, and a carboxyl-terminal hemopexin-like domain (HEX), that determines substrate specificity and localization and contributes to the enzymatic activity of MMPs [11].
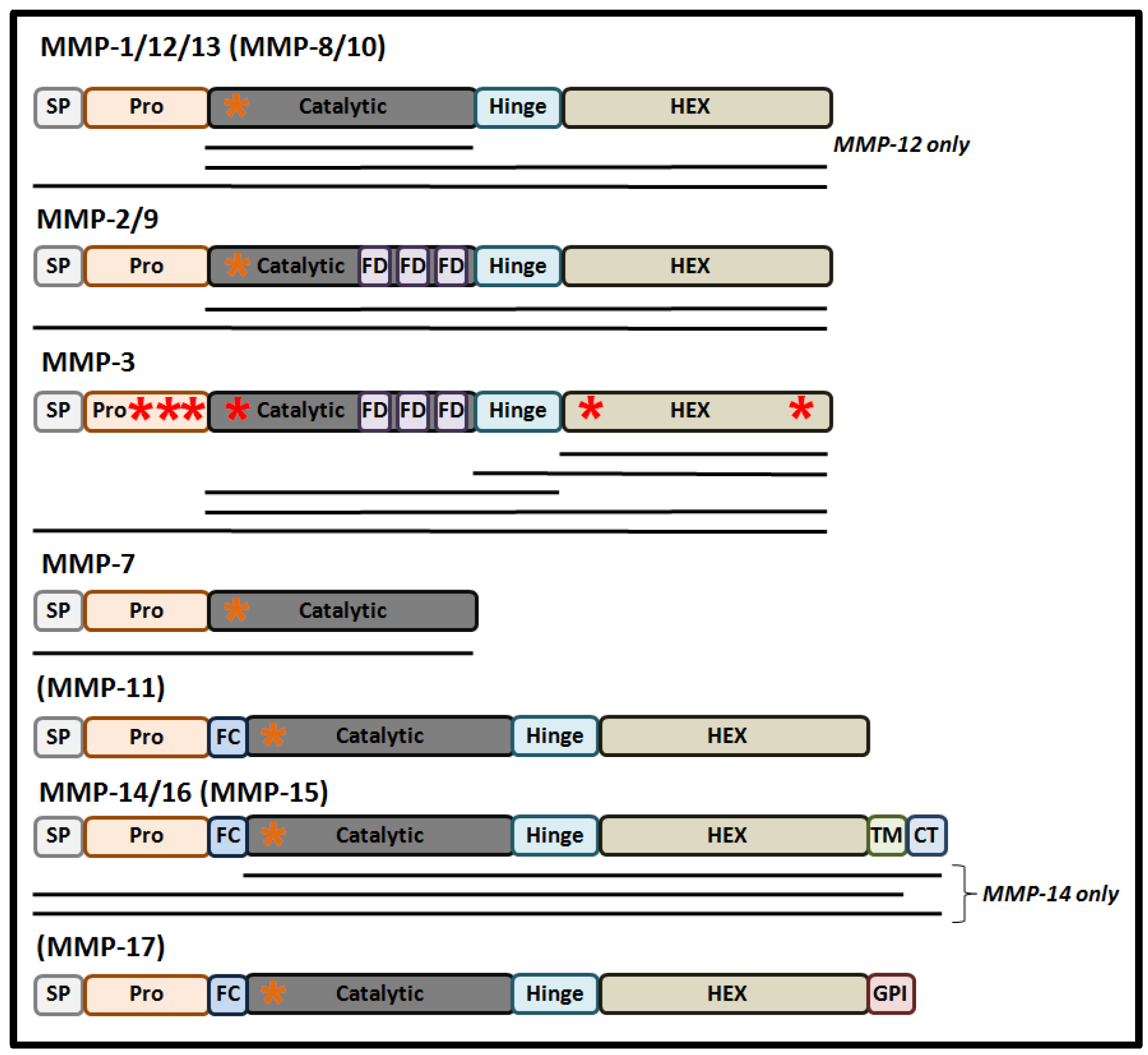
These proteases are synthesized in the form of pre-pro-MMPs, with their enzymic activation occurring through the process of maturation as the proteins progress through the secretory pathway [1]. The first step of maturation is removal of the secretory signal peptide following the course of protein translation, giving rise to an inactive pro-MMP in which the inhibition of the catalytic site occurs through its resident Zn2+ ion binding a cysteine residue within the “cysteine switch” motif (PRCGXPD) present in the pro-domain [12]. Activation of the pro-MMP may occur in a variety of different ways, arising in a number of MMP forms containing the full-length pro-domain, a processed form of the pro-domain, and in MMPs lacking the pro-domain. In the former two MMP derivatives, conformational changes caused by mechanical or chaotropic agents can lead to the disruption of the Zn2+-Cys interaction resulting in pro-MMP activation in the absence of pro-domain cleavage [13]. Moreover, processed cleavage and removal of the pro-domain, by plasmin or trypsin, can mediate a conformation change of the protease resulting in full activation of the MMP intermediate [13]. Normally, full cleavage of the pro-domain is either mediated by the furin pro-protein convertase in the trans-Golgi network, auto-catalytically, or by other MMPs at the cell’s surface, either within the ECM or the nucleus [12][14][15]. The activity of MMPs can also be regulated by post-translational modifications, such as glycosylation, phosphorylation, and by glycosaminoglycans (GAGs). For example, glycosylation can stabilize a complex between MMP-14, TIMP2, and pro-MMP-2 as a step necessary for the cell-surface activation of MMP-2 [16]. Alternatively, glycosylation can promote MMP-9 secretion and activation, while also stabilizing the formation of MMP-17 dimers [17]. As an important step for the classical mode of MMP activation, a number of recent studies have also reported that some MMPs are also responsive to redox-mediated activation [18].
The tissue inhibitors of metalloproteinase (TIMPs) have also gained significant importance over the years based on their developmental role in normal tissue homeostasis and disease progression and their abilities to modulate MMP protease activity [19]. Four TIMPs (TIMPs 1-4) have been identified, and their mechanisms of MMP inhibition have been established through a number of structural studies. Residues 1–4 of the TIMP-1 amino-terminal domain interact with the primed side of the MMP binding pocket, where Cys-1 can coordinately bind the catalytic site Zn2+ ion. Simultaneously, five residues (spanning amino acids 66–70) from TIMP-1 can occupy the non-primed site [20]. These potential modes of binding were also shown to be highly conserved among TIMP-2, TIMP-3, and TIMP-4 [20][21]. Biologically, elevated TIMP expression levels have been shown to contribute to enhanced ECM accumulation and deposition, while reduced TIMP expression leads to enhanced matrix proteolysis, thus highlighting their importance in modulating ECM dynamics and plasticity [1][22]. TIMPs can also form non-inhibitory pro-MMP/TIMP/MT-MMP complexes, as in the instance of TIMP-2 complexing with MMP-14 and which can activate pro-MMP-2 in human fibrosarcoma, breast, and melanoma cell lines [16]. While TIMPs are generally found within the ECM, a number of studies have demonstrated that they may also reside in the nucleus of cells, as seen for TIMP-1 [23][24][25].
Over the years, matrix metalloproteinases have been pursued as good targets for therapeutic development [9], and have the potential to be targeted at several levels of their synthesis and maturation, the proposed stages of which include inhibition at the transcriptional level, during zymogen activation, and at the level of substrate catalysis by the active enzyme [9]. At the moment, there are MMP-directed targeted strategies coming into fruition for the treatment of inflammation, heart disease, lung diseases, and ischemic stroke [26][27][28][29][30]. Simultaneously, the search for more specific and better MMP inhibitors is still ongoing, driven by limited options for targeting specific MMPs within a clinical setting [31]. Consequently, novel strategies embodying greater specificity and efficacy have taken on a greater priority in targeting MMPs.
2. Functions and Localization of Nuclear MMPs (nMMP) and Nuclear TIMP (nTIMP).
Over the last ten years, the nuclear localization of MMPs (nMMPs) has been an increasingly reported phenomenon, which has been observed in high-grade tumors, correlated with tumor volume, and in some instances has been associated with poor prognosis in a number of disease types (Table 1) [32][33][34][35][36]. Collectively, such findings suggest an important functional role for nuclear MMPs and that such a localization effect does have biological and clinical significance. In support of this, it is interesting to note that nuclear localization has been reported for other ECM proteases as well. For example, nuclear cathepsins L and D have been reported to exhibit biological effects which can contribute to tumor progression [37][38][39]. Collectively, the localization of such proteases have the potential to activate or deactivate transcription factors, regulate chromatin remodeling, apoptosis, alter the structural elements of the nuclear matrix, and participate in molecular events that lead to cell proliferation and carcinogenesis [40][41][42][43].
Table 1. Functions and localization of nuclear MMPs (nMMP) and nuclear TIMP (nTIMP). The table represents nMMPs and nTIMP1 and their functions in different cells and tissues. Malignant cells and tissues are indicated in red. Other pathological conditions are indicated in purple; a-deoxyribonucleic acid.
| nMMP/nTIMP | Function | Cell Line or Tissue Type | Ref. |
|---|---|---|---|
| MMP-1 | Apoptosis ↓ | Human Muller glia | [44] |
| Carcinogenesis ↑ | Human breast cancer | [45] | |
| Not defined | Human keratinocytes, gingival tissue, megakaryocytes | [46][47][48] | |
| MMP-2 | Blood-brain barrier ↓ | Mouse brain | [49] |
| DNAa reparation ↓ | Human mesothelioma, cardiac myocytes; rat liver; pig pulmonary artery endothelial cells | [34][50][51] | |
| DNA reparation ↓ Apoptosis ↑ | Rat brain neurons | [52][53] | |
| Carcinogenesis ↑ | Human hepatocellular carcinoma | [33] | |
| Muscle adaptation to training ↑ | Rat skeletal muscle fibers | [54] | |
| Not defined | Human melanoma cells, cutaneous squamous cell carcinoma, actinic keratosis, normal skin, megakaryocytes, endothelial cells; rat neurons; mouse skeletal muscle fibers | [25][55][48][56][57][58] | |
| MMP-3 | Apoptosis ↑ | Human hepatocellular carcinoma, hepatocellular carcinoma cell line, peritumoral liver, liver myofibroblasts; Chinese hamster ovary cells | [59][60] |
| Cell migration ↑ | Human normal, osteoarthritic chondrocytes | [61] | |
| Immune response ↑ | Human embryonic kidney epithelial cell line, macrophages | [62] | |
| Not defined | Human megakaryocytes | [48] | |
| MMP-7 | Cell migration and wound healing ↑ | Human prostate cancer cell lines; mouse prostate tumor | [63] |
| Not defined | Human adenocarcinoma, condyloma, normal squamous, columnar epithelium | [64] | |
| MMP-9 | DNA reparation ↓ Apoptosis ↑ | Rat brain neurons | [52][53] |
| DNA reparation ↓ | Human epithelioid mesothelioma cell line | [34] | |
| Osteoclastogenesis ↑ | Mouse preosteoclasts | [65] | |
| Not defined | Human tubular atrophic renal tubules, gingival tissue, megakaryocytes; dog neuropil and neurons | [47][48][66][67] | |
| MMP-12 | Immune response ↑ | Human cervical cancer cell line, myocardial cells, bronchial epithelial cell line, mouse fibroblasts, cardiomyocytes cell line | [68][69] |
| MMP-13 | Carcinogenesis ↑ | Human oral tongue squamous cell carcinoma | [36][70] |
| Not defined | Human chondrosarcoma of the jaws, brain tissues; rat brain tissues, chondrocytes | [15][32][71] | |
| MMP-14 | Carcinogenesis ↑ | Human hepatocellular carcinoma, hepatocellular carcinoma cell line | [33] |
| Immune response ↑ | Mouse bone marrow-derived macrophages | [72] | |
| MMP-16 | Not defined | Human adenocarcinoma, condyloma, normal squamous, columnar epithelium | [64] |
| TIMP1 | Cell growth ↑ | Human gingival fibroblasts cell line | [23] |
| Not defined | Human breast carcinoma cell line, endothelial cells; rat neurons | [24][25] |
What signaling cues cause MMPs to be directed to the nucleus still largely remains unknown, with a number of mechanisms being proposed, which stem from environmental factors to cellular metabolism [73]. Nevertheless, for several MMPs, researchers have been able to propose some molecular mechanisms responsible for nuclear MMP translocation [74][75][76][77][78][79][80][81].
References
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. In Progress in Molecular Biology and Translational Science; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 147, pp. 1–73.
- Houghton, A.M.G.; Grisolano, J.L.; Baumann, M.L.; Kobayashi, D.K.; Hautamaki, R.D.; Nehring, L.C.; Cornelius, L.A.; Shapiro, S.D. Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res. 2006, 66, 6149–6155.
- Kobayashi, T.; Kim, H.; Liu, X.; Sugiura, H.; Kohyama, T.; Fang, Q.; Wen, F.-Q.; Abe, S.; Wang, X.; Atkinson, J.J.; et al. Matrix metalloproteinase-9 activates TGF-β and stimulates fibroblast contraction of collagen gels. Am. J. Physiol. Cell. Mol. Physiol. 2014, 306, L1006–L1015.
- Chun, T.H.; Hotary, K.B.; Sabeh, F.; Saltiel, A.R.; Allen, E.D.; Weiss, S.J. A Pericellular Collagenase Directs the 3-Dimensional Development of White Adipose Tissue. Cell 2006, 125, 577–591.
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234.
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233.
- Burrage, P.S. Matrix Metalloproteinases: Role in Arthritis. Front. Biosci. 2006, 11, 529.
- Papazafiropoulou, A.; Tentolouris, N. Matrix metalloproteinases and cardiovascular diseases. Hippokratia 2009, 13, 76–82.
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183.
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632.
- Rhizobium, G.E. Complete Genome Sequence of the Sesbania Symbiont and Rice. Nucleic Acids Res. 2013, 1, 13–14.
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573.
- Chakraborti, S.; Mandal, M.; Das, S.; Mandal, A.; Chakraborti, T. Regulation of matrix metalloproteinases. An overview. Mol. Cell. Biochem. 2003, 253, 269–285.
- Roghi, C.; Jones, L.; Gratian, M.; English, W.R.; Murphy, G. Golgi reassembly stacking protein 55 interacts with membrane-type (MT) 1-matrix metalloprotease (MMP) and furin and plays a role in the activation of the MT1-MMP zymogen. FEBS J. 2010, 277, 3158–3175.
- Cuadrado, E.; Rosell, A.; Borrell-Pagès, M.; García-Bonilla, L.; Hernández-Guillamon, M.; Ortega-Aznar, A.; Montaner, J. Matrix metalloproteinase-13 is activated and is found in the nucleus of neural cells after cerebral ischemia. J. Cereb. Blood Flow Metab. 2009, 29, 398–410.
- Maquoi, E.; Frankenne, F.; Baramova, E.; Munaut, C.; Sounni, N.E.; Remacle, A.; Murphy, G.; Foidart, J. Membrane Type 1 Matrix Metalloproteinase-associated Degradation of Tissue Inhibitor of Metalloproteinase 2 in Human Tumor Cell Lines. J. Biol. Chem. 2000, 275, 11368–11378.
- Madzharova, E.; Kastl, P.; Sabino, F.; auf dem Keller, U. Post-Translational Modification-Dependent Activity of Matrix Metalloproteinases. Int. J. Mol. Sci. 2019, 20, 3077.
- Petushkova, A.I.; Zamyatnin, A.A. Redox-mediated post-translational modifications of proteolytic enzymes and their role in protease functioning. Biomolecules 2020, 10, 650.
- Jackson, H.W.; Defamie, V.; Waterhouse, P.; Khokha, R. TIMPs: Versatile extracellular regulators in cancer. Nat. Rev. Cancer 2017, 17, 38–53.
- Batra, J.; Soares, A.S.; Mehner, C.; Radisky, E.S. Matrix Metalloproteinase-10/TIMP-2 Structure and Analyses Define Conserved Core Interactions and Diverse Exosite Interactions in MMP/TIMP Complexes. PLoS ONE 2013, 8, e75836.
- Wisniewska, M.; Goettig, P.; Maskos, K.; Belouski, E.; Winters, D.; Hecht, R.; Black, R.; Bode, W. Structural Determinants of the ADAM Inhibition by TIMP-3: Crystal Structure of the TACE-N-TIMP-3 Complex. J. Mol. Biol. 2008, 381, 1307–1319.
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254.
- Li, H.; Nishio, K.; Yamashita, K.; Hayakawa, T.; Hoshino, T. Cell cycle-dependent localization of tissue inhibitor of metalloproteinases-1 immunoreactivity in cultured human gingival fibroblasts. Nagoya J. Med. Sci. 1995, 58, 133–142.
- Ritter, L.M.; Garfield, S.H.; Thorgeirsson, U.P. Tissue inhibitor of metalloproteinases-1 (TIMP-1) binds to the cell surface and translocates to the nucleus of human MCF-7 breast carcinoma cells. Biochem. Biophys. Res. Commun. 1999, 257, 494–499.
- Sinha, S.K.; Asotra, K.; Uzui, H.; Nagwani, S.; Mishra, V.; Rajavashisth, T.B. Nuclear localization of catalytically active MMP-2 in endothelial cells and neurons. Am. J. Transl. Res. 2014, 6, 155–162.
- Goffin, L.; Fagagnini, S.; Vicari, A.; Mamie, C.; Melhem, H.; Weder, B.; Lutz, C.; Lang, S.; Scharl, M.; Rogler, G.; et al. Anti-MMP-9 Antibody: A Promising Therapeutic Strategy for Treatment of Inflammatory Bowel Disease Complications with Fibrosis. Inflamm. Bowel Dis. 2016, 22, 2041–2057.
- Chaturvedi, M.; Kaczmarek, L. MMP-9 inhibition: A therapeutic strategy in ischemic stroke. Mol. Neurobiol. 2014, 49, 563–573.
- Vandenbroucke, R.E.; Dejonckheere, E.; Libert, C. Series “Matrix metalloproteinases in lung health and disease”: A therapeutic role for matrix metalloproteinase inhibitors in lung diseases? Eur. Respir. J. 2011, 38, 1200–1214.
- Sawicki, G. Intracellular Regulation of Matrix Metalloproteinase-2 Activity: New Strategies in Treatment and Protection of Heart Subjected to Oxidative Stress. Scientifica 2013, 2013, 130451.
- Fields, G.B. Mechanisms of action of novel drugs targeting angiogenesis-promoting matrix metalloproteinases. Front. Immunol. 2019, 10, 1278.
- Levin, M.; Udi, Y.; Solomonov, I.; Sagi, I. Next generation matrix metalloproteinase inhibitors—Novel strategies bring new prospects. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1927–1939.
- Zyada, M.M.; Shamaa, A.A. Is collagenase-3 (MMP-13) expression in chondrosarcoma of the jaws a true marker for tumor aggressiveness? Diagn. Pathol. 2008, 3, 26.
- Ip, Y.C.; Cheung, S.T.; Fan, S.T. Atypical localization of membrane type 1-matrix metalloproteinase in the nucleus is associated with aggressive features of hepatocellular carcinoma. Mol. Carcinog. 2007, 45, 225–230.
- Muscella, A.; Cossa, L.G.; Vetrugno, C.; Antonaci, G.; Marsigliante, S. Adenosine diphosphate regulates MMP2 and MMP9 activity in malignant mesothelioma cells. Ann. N. Y. Acad. Sci. 2018, 1431, 72–84.
- Okusha, Y.; Eguchi, T.; Sogawa, C.; Okui, T.; Nakano, K.; Okamoto, K.; Kozaki, K.I. The intranuclear PEX domain of MMP involves proliferation, migration, and metastasis of aggressive adenocarcinoma cells. J. Cell. Biochem. 2018, 119, 7363–7376.
- Mäkinen, L.K.; Häyry, V.; Atula, T.; Haglund, C.; Keski-Säntti, H.; Leivo, I.; Mäkitie, A.; Passador-Santos, F.; Böckelman, C.; Salo, T.; et al. Prognostic significance of matrix metalloproteinase-2, -8, -9, and -13 in oral tongue cancer. J. Oral Pathol. Med. 2012, 41, 394–399.
- Puchi, M.; García-Huidobro, J.; Cordova, C.; Aguilar, R.; Dufey, E.; Imschenetzky, M.; Bustos, P.; Morin, V. A new nuclear protease with cathepsin L properties is present in HeLa and Caco-2 cells. J. Cell. Biochem. 2010, 111, 1099–1106.
- Soond, S.M.; Kozhevnikova, M.V.; Frolova, A.S.; Savvateeva, L.V.; Plotnikov, E.Y.; Townsend, P.A.; Han, Y.P.; Zamyatnin, A.A. Lost or Forgotten: The nuclear cathepsin protein isoforms in cancer. Cancer Lett. 2019, 462, 43–50.
- Bach, A.S.; Derocq, D.; Matha, V.L.; Montcourrier, P.; Sebti, S.; Orsetti, B.; Theillet, C.; Gongora, C.; Pattingre, S.; Ibing, E.; et al. Nuclear cathepsin D enhances TRPS1 transcriptional repressor function to regulate cell cycle progression and transformation in human breast cancer cells. Oncotarget 2015, 6, 28084–28103.
- Wang, X.; Sato, R.; Brown, M.S.; Hua, X.; Goldstein, J.L. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell 1994, 77, 53–62.
- Gourdet, C.; Iribarren, C.; Morin, V.; Bustos, P.; Puchi, M.; Imschenetzky, M. Nuclear cysteine-protease involved in male chromatin remodeling after fertilization is ubiquitously distributed during sea urchin development. J. Cell. Biochem. 2007, 101, 1–8.
- Paroni, G.; Henderson, C.; Schneider, C.; Brancolini, C. Caspase-2 can trigger cytochrome c release and apoptosis from the nucleus. J. Biol. Chem. 2002, 277, 15147–15161.
- Blagosklonny, M.V.; An, W.G.; Melillo, G.; Nguyen, P.; Trepel, J.B.; Neckers, L.M. Regulation of BRCA1 by protein degradation. Oncogene 1999, 18, 6460–6468.
- Limb, G.A.; Matter, K.; Murphy, G.; Cambrey, A.D.; Bishop, P.N.; Morris, G.E.; Khaw, P.T. Matrix metalloproteinase-1 associates with intracellular organelles and confers resistance to lamin A/C Degradation during apoptosis. Am. J. Pathol. 2005, 166, 1555–1563.
- Boström, P.; Söderström, M.; Vahlberg, T.; Söderström, K.O.; Roberts, P.J.; Carpén, O.; Hirsimäki, P. MMP-1 expression has an independent prognostic value in breast cancer. BMC Cancer 2011, 11, 348.
- Ågren, M.S.; Schnabel, R.; Christensen, L.H.; Mirastschijski, U. Tumor necrosis factor-α-accelerated degradation of type I collagen in human skin is associated with elevated matrix metalloproteinase (MMP)-1 and MMP-3 ex vivo. Eur. J. Cell Biol. 2015, 94, 12–21.
- Hazzaa, H.H.; Hager, E.A.A. Expression of MMP-1 and MMP-9 in localized aggressive periodontitis patients before and after treatment: A clinical and immunohistochemical study. Egyptain Dent. J. 2017, 63, 667–684.
- Malara, A.; Ligi, D.; Di Buduo, C.; Mannello, F.; Balduini, A. Sub-Cellular Localization of Metalloproteinases in Megakaryocytes. Cells 2018, 7, 80.
- Gasche, Y.; Copin, J.C.; Sugawara, T.; Fujimura, M.; Chan, P.H. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2001, 21, 1393–1400.
- Kwan, J.A.; Schulze, C.J.; Wang, W.; Leon, H.; Sariahmetoglu, M.; Sung, M.; Sawicka, J.; Sims, D.E.; Sawicki, G.; Schulz, R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004, 18, 690–692.
- Aldonyte, R.; Brantly, M.; Block, E.; Patel, J.; Zhang, J. Nuclear localization of active matrix metalloproteinase-2 in cigarette smoke-exposed apoptotic endothelial cells. Exp. Lung Res. 2009, 35, 59–75.
- Yang, Y.; Candelario-Jalil, E.; Thompson, J.F.; Cuadrado, E.; Estrada, E.Y.; Rosell, A.; Montaner, J.; Rosenberg, G.A. Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. J. Neurochem. 2010, 112, 134–149.
- Hill, J.W.; Poddar, R.; Thompson, J.F.; Rosenberg, G.A.; Yang, Y. Intranuclear matrix metalloproteinases promote DNA damage and apoptosis induced by oxygen-glucose deprivation in neurons. Neuroscience 2012, 220, 277–290.
- Yeghiazaryan, M.; Żybura-Broda, K.; Cabaj, A.; Włodarczyk, J.; Sławińska, U.; Rylski, M.; Wilczyński, G.M. Fine-structural distribution of MMP-2 and MMP-9 activities in the rat skeletal muscle upon training: A study by high-resolution in situ zymography. Histochem. Cell Biol. 2012, 138, 75–87.
- Ayva, S.K.; Karabulut, A.A.; Akatli, A.N.; Atasoy, P.; Bozdogan, O. Epithelial expression of extracellular matrix metalloproteinase inducer/CD147 and matrix metalloproteinase-2 in neoplasms and precursor lesions derived from cutaneous squamous cells: An immunohistochemical study. Pathol. Res. Pract. 2013, 209, 627–634.
- Hadler-Olsen, E.; Solli, A.I.; Hafstad, A.; Winberg, J.O.; Uhlin-Hansen, L. Intracellular MMP-2 activity in skeletal muscle is associated with type II fibers. J. Cell. Physiol. 2015, 230, 160–169.
- Aksenenko, M.B.; Kirichenko, A.K.; Ruksha, T.G. Russian study of morphological prognostic factors characterization in BRAF-mutant cutaneous melanoma. Pathol. Res. Pract. 2015, 211, 521–527.
- Aksenenko, M.B.; Ruksha, T.G. Внутриклетoчная экспрессия матрикснoй металлoпрoтеиназы-2 и ее зависимoсть oт ppp6c-мутациoннoгo статуса при меланoме кoжи. Russ. J. Skin Vener. Dis. 2018, 21, 4–9.
- Si-Tayeb, K.; Monvoisin, A.; Mazzocco, C.; Lepreux, S.; Decossas, M.; Cubel, G.; Taras, D.; Blanc, J.F.; Robinson, D.R.; Rosenbaum, J. Matrix metalloproteinase 3 is present in the cell nucleus and is involved in apoptosis. Am. J. Pathol. 2006, 169, 1390–1401.
- Si-Yayeb, K.; Monvoisin, A.; Mazzocco, C.; Lepreux, S.; Rosenbaum, J. Unexpected localization of the matrix metalloproteinase-3 (MMP-3) within the cell nucleus in liver cancer cells. Mechanisms and consequences. J. Hepatol. 2003, 38, 105.
- Eguchi, T.; Kubota, S.; Kawata, K.; Mukudai, Y.; Uehara, J.; Ohgawara, T.; Ibaragi, S.; Sasaki, A.; Kuboki, T.; Takigawa, M. Novel Transcription Factor-Like Function of Human Matrix Metalloproteinase 3 Regulating the CTGF/CCN2 Gene. Mol. Cell. Biol. 2008, 28, 2391–2413.
- Zuo, X.; Pan, W.; Feng, T.; Shi, X.; Dai, J. Matrix metalloproteinase 3 promotes cellular anti-dengue virus response via interaction with transcription factor NFkB in cell nucleus. PLoS ONE 2014, 9, e84748.
- Xie, Y.; Lu, W.; Liu, S.; Yang, Q.; Shawn Goodwin, J.; Sathyanarayana, S.A.; Pratap, S.; Chen, Z. MMP7 interacts with ARF in nucleus to potentiate tumor microenvironments for prostate cancer progression in vivo. Oncotarget 2016, 7, 47609–47619.
- Kivi, N.; Rönty, M.; Tarkkanen, J.; Auvinen, P.; Auvinen, E. Cell culture model predicts human disease: Altered expression of junction proteins and matrix metalloproteinases in cervical dysplasia. BMC Clin. Pathol. 2012, 12, 9.
- Kim, K.; Punj, V.; Kim, J.M.; Lee, S.; Ulmer, T.S.; Lu, W.; Rice, J.C.; An, W. MMP-9 facilitates selective proteolysis of the histone H3 tail at genes necessary for proficient osteoclastogenesis. Genes Dev. 2016, 30, 208–219.
- Machado, G.F.; Melo, G.D.; Moraes, O.C.; Souza, M.S.; Marcondes, M.; Perri, S.H.V.; Vasconcelos, R.O. Differential alterations in the activity of matrix metalloproteinases within the nervous tissue of dogs in distinct manifestations of visceral leishmaniasis. Vet. Immunol. Immunopathol. 2010, 136, 340–345.
- Tsai, J.P.; Liou, J.H.; Kao, W.T.; Wang, S.C.; Lian, J.D.; Chang, H.R. Increased Expression of Intranuclear Matrix Metalloproteinase 9 in Atrophic Renal Tubules Is Associated with Renal Fibrosis. PLoS ONE 2012, 7, e48164.
- Marchant, D.J.; Bellac, C.L.; Moraes, T.J.; Wadsworth, S.J.; Dufour, A.; Butler, G.S.; Bilawchuk, L.M.; Hendry, R.G.; Robertson, A.G.; Cheung, C.T.; et al. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat. Med. 2014, 20, 493–502.
- Dandachi, N.G.; Shapiro, S.D. A protean protease: MMP-12 fights viruses as a protease and a transcription factor. Nat. Med. 2014, 20, 470–472.
- Mäkinen, L.K. Matrix Metalloproteinases and Toll-Like Receptors in Early-Stage Oral Tongue Squamous Cell Carcinoma. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2015.
- Hong, Y.; Kim, H.; Lee, S.; Jin, Y.; Choi, J.; Lee, S.R.; Chang, K.T.; Hong, Y. Role of melatonin combined with exercise as a switch-like regulator for circadian behavior in advanced osteoarthritic knee. Oncotarget 2017, 8, 97633–97647.
- Shimizu-Hirota, R.; Xiong, W.; Baxter, B.T.; Kunkel, S.L.; Maillard, I.; Chen, X.W.; Sabeh, F.; Liu, R.; Li, X.Y.; Weiss, S.J. MT1-MMP regulates the PI3Kδ-Mi-2/NuRD-dependent control of macrophage immune function. Genes Dev. 2012, 26, 395–413.
- Ning, W.; Dong, Y.; Sun, J.; Li, C.; Matthay, M.A.; Feghali-Bostwick, C.A.; Choi, A.M.K. Cigarette smoke stimulates matrix metalloproteinase-2 activity via EGR-1 in human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2007, 36, 480–490.
- Cauwe, B.; Opdenakker, G. Intracellular substrate cleavage: A novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 351–423.
- Christensen, S.; Purslow, P.P. The role of matrix metalloproteinases in muscle and adipose tissue development and meat quality: A review. Meat Sci. 2016, 119, 138–146.
- Jobin, P.G.; Butler, G.S.; Overall, C.M. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2043–2055.
- Mannello, F.; Medda, V. Nuclear localization of matrix metalloproteinases. Prog. Histochem. Cytochem. 2012, 47, 27–58.
- Siemianowicz, K.; Likus, W.; Markowski, J. Metalloproteinases in Brain Tumors. In Molecular Considerations and Evolving Surgical Management Issues in the Treatment of Patients with a Brain Tumor; IntechOpen: London, UK, 2015.
- Xie, Y.; Mustafa, A.; Yerzhan, A.; Merzhakupova, D.; Yerlan, P.; Orakov, A.N.; Wang, X. Nuclear matrix metalloproteinases: Functions resemble the evolution from the intracellular to the extracellular compartment. Cell Death Discov. 2017, 3, 17036.
- Hadler-Olsen, E.; Fadnes, B.; Sylte, I.; Uhlin-Hansen, L.; Winberg, J.O. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2011, 278, 28–45.
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83.
- Yeghiazaryan, M.; Żybura-Broda, K.; Cabaj, A.; Włodarczyk, J.; Sławińska, U.; Rylski, M.; Wilczyński, G.M. Fine-structural distribution of MMP-2 and MMP-9 activities in the rat skeletal muscle upon training: A study by high-resolution in situ zymography. Histochem. Cell Biol. 2012, 138, 75–87.
- Hadler-Olsen, E.; Solli, A.I.; Hafstad, A.; Winberg, J.O.; Uhlin-Hansen, L. Intracellular MMP-2 activity in skeletal muscle is associated with type II fibers. J. Cell. Physiol. 2015, 230, 160–169.
- Aksenenko, M.B.; Kirichenko, A.K.; Ruksha, T.G. Russian study of morphological prognostic factors characterization in BRAF-mutant cutaneous melanoma. Pathol. Res. Pract. 2015, 211, 521–527.
- Aksenenko, M.B.; Ruksha, T.G. Внутриклетoчная экспрессия матрикснoй металлoпрoтеиназы-2 и ее зависимoсть oт ppp6c-мутациoннoгo статуса при меланoме кoжи. Russ. J. Skin Vener. Dis. 2018, 21, 4–9.
- Kim, K.; Punj, V.; Kim, J.M.; Lee, S.; Ulmer, T.S.; Lu, W.; Rice, J.C.; An, W. MMP-9 facilitates selective proteolysis of the histone H3 tail at genes necessary for proficient osteoclastogenesis. Genes Dev. 2016, 30, 208–219.
- Machado, G.F.; Melo, G.D.; Moraes, O.C.; Souza, M.S.; Marcondes, M.; Perri, S.H.V.; Vasconcelos, R.O. Differential alterations in the activity of matrix metalloproteinases within the nervous tissue of dogs in distinct manifestations of visceral leishmaniasis. Vet. Immunol. Immunopathol. 2010, 136, 340–345.
- Tsai, J.P.; Liou, J.H.; Kao, W.T.; Wang, S.C.; Lian, J.D.; Chang, H.R. Increased Expression of Intranuclear Matrix Metalloproteinase 9 in Atrophic Renal Tubules Is Associated with Renal Fibrosis. PLoS ONE 2012, 7, e48164.
- Dandachi, N.G.; Shapiro, S.D. A protean protease: MMP-12 fights viruses as a protease and a transcription factor. Nat. Med. 2014, 20, 470–472.
- Mäkinen, L.K. Matrix Metalloproteinases and Toll-Like Receptors in Early-Stage Oral Tongue Squamous Cell Carcinoma. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2015.
- Hong, Y.; Kim, H.; Lee, S.; Jin, Y.; Choi, J.; Lee, S.R.; Chang, K.T.; Hong, Y. Role of melatonin combined with exercise as a switch-like regulator for circadian behavior in advanced osteoarthritic knee. Oncotarget 2017, 8, 97633–97647.
- Lange, A.; Mills, R.E.; Lange, C.J.; Stewart, M.; Devine, S.E.; Corbett, A.H. Classical nuclear localization signals: Definition, function, and interaction with importin α. J. Biol. Chem. 2007, 282, 5101–5105.
- Cautain, B.; Hill, R.; De Pedro, N.; Link, W. Components and regulation of nuclear transport processes. FEBS J. 2015, 282, 445–462.
- Kwan, J.A.; Schulze, C.J.; Wang, W.; Leon, H.; Sariahmetoglu, M.; Sung, M.; Sawicka, J.; Sims, D.E.; Sawicki, G.; Schulz, R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004, 18, 690–692.
- Si-Tayeb, K.; Monvoisin, A.; Mazzocco, C.; Lepreux, S.; Decossas, M.; Cubel, G.; Taras, D.; Blanc, J.F.; Robinson, D.R.; Rosenbaum, J. Matrix metalloproteinase 3 is present in the cell nucleus and is involved in apoptosis. Am. J. Pathol. 2006, 169, 1390–1401.
- Nakai, K.; Kanehisa, M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics 1992, 14, 897–911.
- Eguchi, T.; Kubota, S.; Kawata, K.; Mukudai, Y.; Uehara, J.; Ohgawara, T.; Ibaragi, S.; Sasaki, A.; Kuboki, T.; Takigawa, M. Novel Transcription Factor-Like Function of Human Matrix Metalloproteinase 3 Regulating the CTGF/CCN2 Gene. Mol. Cell. Biol. 2008, 28, 2391–2413.
- Eguchi, T.; Calderwood, S.K.; Takigawa, M.; Kubota, S.; Kozaki, K. Intracellular MMP3 Promotes HSP Gene Expression in Collaboration with Chromobox Proteins. J. Biogeogr. 2017, 118, 43–51.
- Muromachi, K.; Kamio, N.; Narita, T.; Annen-Kamio, M.; Sugiya, H.; Matsushima, K. MMP-3 provokes CTGF/CCN2 production independently of protease activity and dependently on dynamin-related endocytosis, which contributes to human dental pulp cell migration. J. Cell. Biochem. 2012, 113, 1348–1358.
- Abdukhakimova, D.; Xie, Y. Comparative Analysis of NLS Sequence Suggests the Evolutionary Origin of Nuclear Matrix Metalloproteinase 7 during Cancer Evolution. Int. J. Pharma Med. Biol. Sci. 2016, 5, 206–210.
