LIN28 inhibits
let-7
miRNA maturation which prevents cell differentiation and promotes proliferation. We hypothesized that the LIN28-
let-7
axis regulates proliferation-associated genes in sheep trophectoderm in vivo. Day 9-hatched sheep blastocysts were incubated with lentiviral particles to deliver shRNA targeting LIN28 specifically to trophectoderm cells. At day 16, conceptus elongation was significantly reduced in LIN28A and LIN28B knockdowns.
Let-7
miRNAs were significantly increased and IGF2BP1-3, HMGA1, ARID3B, and c-MYC were decreased in trophectoderm from knockdown conceptuses. Ovine trophoblast (OTR) cells derived from day 16 trophectoderm are a useful tool for in vitro experiments. Surprisingly, LIN28 was significantly reduced and
let-7
miRNAs increased after only a few passages of OTR cells, suggesting these passaged cells represent a more differentiated phenotype. To create an OTR cell line more similar to day 16 trophectoderm we overexpressed LIN28A and LIN28B, which significantly decreased
let-7
miRNAs and increased IGF2BP1-3, HMGA1, ARID3B, and c-MYC compared to control. This is the first study showing the role of the LIN28-
let-7
axis in trophoblast proliferation and conceptus elongation in vivo. These results suggest that reduced LIN28 during early placental development can lead to reduced trophoblast proliferation and sheep conceptus elongation at a critical period for successful establishment of pregnancy.
- Blastocyst
- Conceptus Elongation
- Proliferation
1.LIN28 Knockdown Introducin Trophectoderm Resulted in Reduced Proliferation of Trophoblast Cells and Lower Expression of Proliferation-Associated Genes
1.1. LIN28 Knockdown in Trophectoderm Resulted in Reduced Proliferation of Trophoblast Cells and Lower Expression of Proliferation-Associated Genes
Day 9-hatched blastocysts were infected with lentiviral particles expressing shRNA to knockdown LIN28A (AKD) or LIN28B (BKD), or scramble control shRNA (SC). The blastocysts treated with lentiviral particles were surgically transferred to synchronized ewes at day 9 of estrus. The conceptuses were collected at day 16 of gestation and trophectoderm (TE) was separated from embryo. LIN28A and LIN28B mRNAs and proteins were quantified by real-time PCR and Western blot. LIN28A mRNA and protein was significantly reduced in AKD TE while LIN28B mRNA and protein was significantly reduced in BKD TE compared to SC (Figure 1A,B). As expected, due to reduced LIN28, let-7 miRNAs (let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, let-7i) were significantly higher in AKD and BKD TE compared to SC, and there was no significant change in let-7 miRNAs between AKD and BKD TE (Figure 1C). These results suggest that reduced LIN28A or LIN28B led to significant increase in let-7 miRNAs.
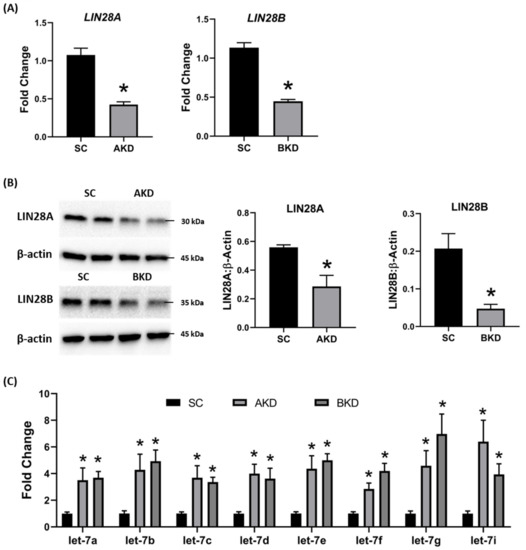
Figure 1. LIN28A or LIN28B knockdown and let-7 miRNAs in day 16 sheep TE. (A) LIN28A and LIN28B mRNA in AKD (n = 5) and BKD (n = 6) day 16 TE compared to SC (n = 6). (B) Representative immunoblots for LIN28A, LIN28B, and β-actin in AKD, BKD, and SC day 16 TE, and densitometric analysis. (C) Let-7 miRNAs in AKD and BKD day 16 TE and SC. * p < 0.05 vs. SC.
To determine the effect of LIN28 knockdown on conceptus elongation, we measured the length of TE. Knockdown of LIN28A or LIN28B resulted in a significant reduction in day 16 TE length compared to SC (Figure 2A,B). There was no significant difference in elongation of AKD vs. BKD TE. This data suggest knockdown of either LIN28A or LIN28B in vivo resulted in reduced proliferation of trophoblast cells.
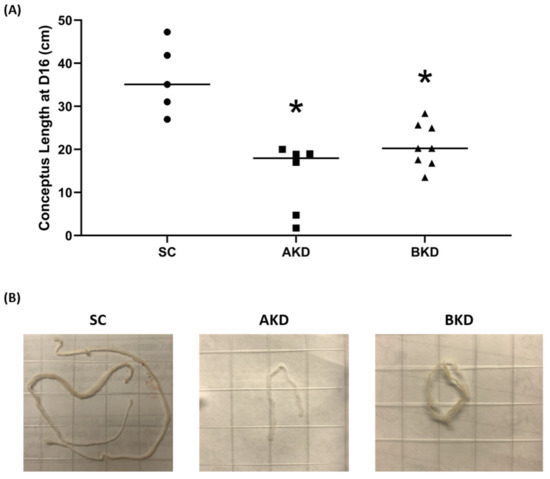
Figure 2. (A) Conceptus length at day 16 after LIN28A knockdown (AKD, n = 6) and LIN28B knockdown (BKD, n = 8) compared to scramble control (SC, n = 5). (B) Representative images of day 16 sheep conceptuses for AKD, BKD, and SC day 16 TE; * p < 0.05 vs. SC.
Due to the potential for reduced proliferation of trophoblast cells in the AKD and BKD conceptuses, we measured the mRNA and protein levels of let-7-regulated proliferation-associated genes. The mRNA and protein levels of IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC were significantly reduced in AKD and BKD day 16 TE compared to SC (Figure 3, Figure 4). These results suggest that high let-7 miRNAs in AKD and BKD TE led to a significant reduction in expression of IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC, and the reduced proliferation of trophoblast cells in AKD and BKD TE is due to significantly reduced expression of these proliferation-associated genes.
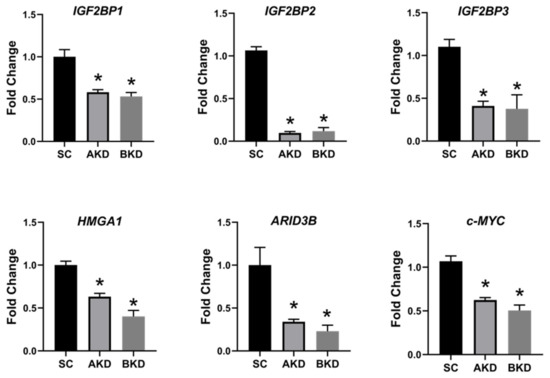
Figure 3. IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC mRNA in AKD and BKD day 16 TE compared to SC (n = 5), * p < 0.05 vs. SC.
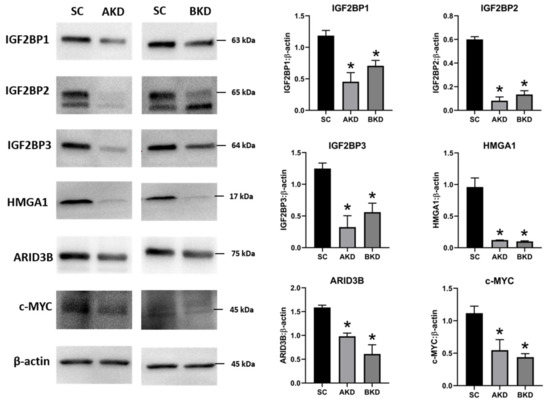
Figure 4. Representative immunoblots for IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, c-MYC, and β-actin, and densitometric analysis of immunoblotting results in AKD and BKD day 16 sheep TE compared to SC (n = 3), * p < 0.05 vs. SC.
2. Ovine Trophoblast Cells Generated from Day 16 Trophectoderm Had a Significant Reduction in LIN28
To further investigate the regulation of ovine trophoblast cell proliferation by LIN28 in vitro, we used day 16 TE to generate ovine trophoblast cells. The day 16 TE was minced and plated in collagen-coated plates and was passaged to obtain a cell line. The cells used for further experiments were collected at passage 4–6, so we called these cells non-immortalized ovine trophoblast (OTR) cells. Interestingly, real-time PCR data showed that OTR cells had a significant reduction in LIN28A and LIN28B mRNAs compared to day 16 TE (Figure 5A). Densitometric analysis of Western blots showed that LIN28A and LIN28B proteins were also significantly reduced in OTR cells compared to day 16 TE (Figure 5B). Furthermore, real-time PCR data showed significant increase in let-7 miRNAs (let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, let-7i) in OTR cells compared to day 16 TE (Figure 5C). The significantly reduced LIN28 and high let-7 miRNAs in OTR cells after only 4–6 passages suggest that these cells differentiated to a different phenotype compared to trophoblast cells in day 16 TE.
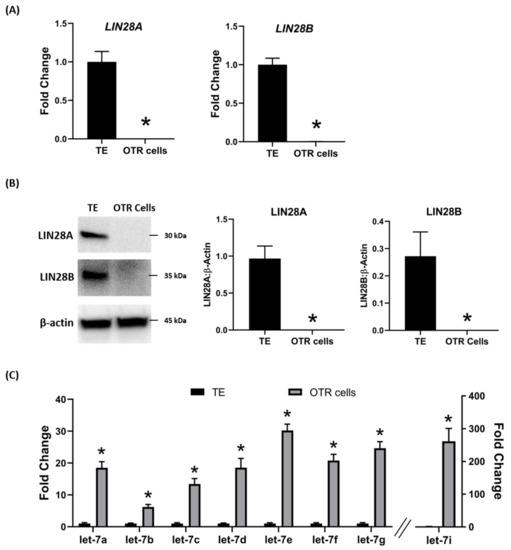
Figure 5. LIN28A, LIN28B, and let-7 miRNAs in non-immortalized ovine trophoblast (OTR) cells. (A) LIN28A and LIN28B mRNA in OTR cells compared to day 16 sheep TE. (B) Representative immunoblots for LIN28A, LIN28B, and β-actin, and densitometric analysis of immunoblotting results in OTR cells compared to day 16 TE. (C) Let-7 miRNAs in OTR cells compared to day 16 TE (n = 3), * p < 0.05 vs. TE.
The effect of low LIN28 and high let-7 miRNAs on proliferation-associated genes in OTR cells was determined by measuring IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC mRNAs and proteins. Real-time PCR showed that mRNA levels of IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC were significantly reduced in OTR cells compared to day 16 TE (Figure 6). Densitometric analysis of Western blots revealed a significant reduction in protein levels of IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC in OTR cells compared to day 16 TE (Figure 7). These results suggest that reduced LIN28 and high let-7 miRNAs led to reduced expression of proliferation-associated genes in OTR cells.
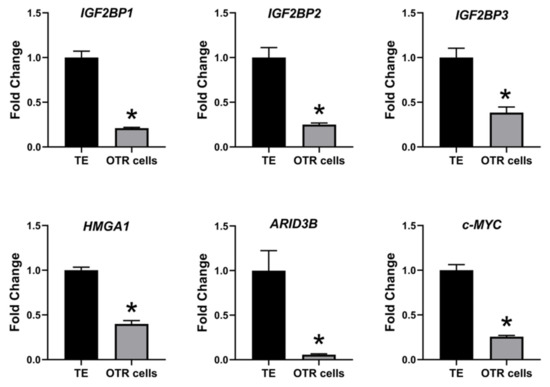
Figure 6. IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC mRNA in OTR cells compared to day 16 TE (n = 3), * p < 0.05 vs. TE.
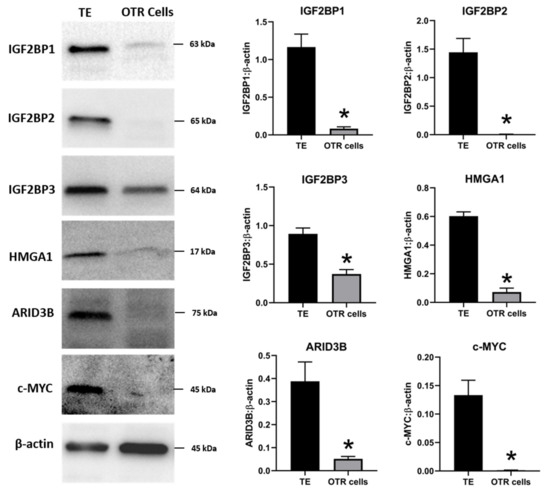
Figure 7. Representative immunoblots for IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, c-MYC, and β-actin, and densitometric analysis of immunoblotting results in OTR cells compared to day 16 TE (n = 3), * p < 0.05 vs. TE.
The OTR cells originated from day 16 TE undergo senescence after only a few passages. Therefore, the OTR cells were immortalized by overexpressing human telomerase reverse transcriptase (hTERT) to keep them growing for further in vitro experiments. The newly generated immortalized cells were referred to as immortalized ovine trophoblast (iOTR) cells.
3. Overexpression of LIN28 in iOTR Cells Resulted in Increased Expression of Proliferation-Associated Genes
To determine if LIN28 overexpression will rescue the expression of proliferation-associated genes, the iOTR cells were infected with lentiviral particles to generate LIN28A knockin (AKI) or LIN28B knockin (BKI), or lentiviral particles with empty expression vector as expression vector control (EVC). Real-time PCR data showed that AKI iOTR cells had a significant increase in LIN28A mRNA while BKI iOTR cells had a significant increase in LIN28B mRNA compared to EVC (Figure 8A). The densitometric analysis of Western blots showed a significant increase in LIN28A protein in AKI and significant increase in LIN28B protein in BKI iOTR cells compared to EVC (Figure 8B). Moreover, the real-time PCR data showed that let-7 miRNAs (let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, let-7i) were significantly reduced in both AKI and BKI iOTR cells compared to EVC (Figure 8C). These results suggest that increased expression of either LIN28A or LIN28B leads to reduction in let-7 miRNAs.
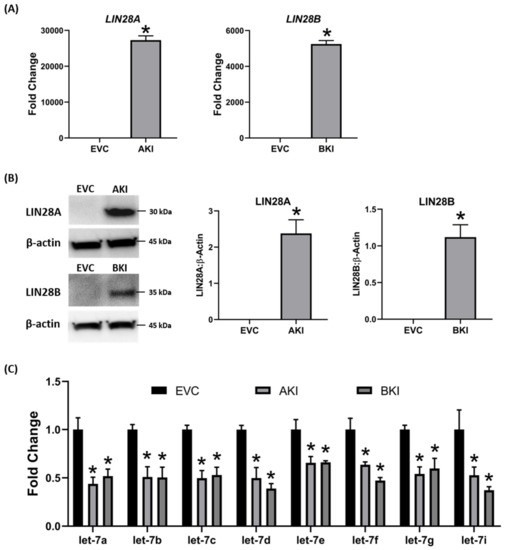
Figure 8. LIN28A, LIN28B, and let-7 miRNAs in LIN28A knockin (AKI) and LIN28B knockin (BKI) immortalized ovine trophoblast (iOTR) cells. (A) LIN28A and LIN28B mRNA in AKI and BKI iOTR cells compared to expression vector control (EVC). (B) Representative immunoblots for LIN28A, LIN28B, and β-actin, and densitometric analysis of immunoblotting results in AKI and BKI iOTR cells compared to EVC. (C) Let-7 miRNAs in AKI and BKI iOTR cells compared to EVC (n = 3), * p < 0.05 vs. EVC.
To determine the effect of LIN28 overexpression and reduction in let-7 miRNAs on expression of proliferation-associated genes, real-time PCR and Western blot analysis were done. Real-time PCR showed that mRNA levels of IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC were significantly increased in both AKI and BKI iOTR cells compared to EVC (Figure 9). Densitometric analysis of Western blots showed significant increase in IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC proteins in both AKI and BKI iOTR cells compared to EVC (Figure 10). These results suggest that the expression of proliferation-associated genes in immortalized ovine trophoblast cells is regulated by the LIN28-let-7 axis.
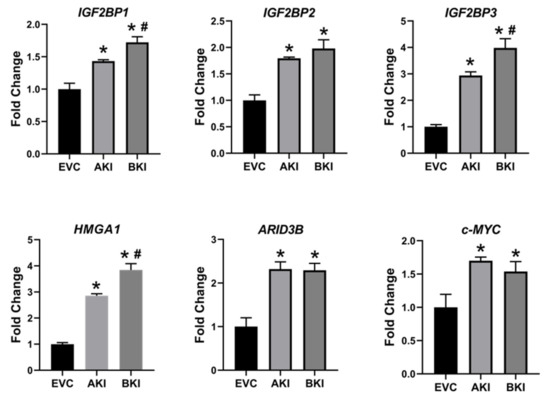
Figure 9. IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC mRNA in AKI and BKI iOTR cells compared to EVC (n = 3), where * p < 0.05 vs. EVC and # p < 0.05 vs AKI.
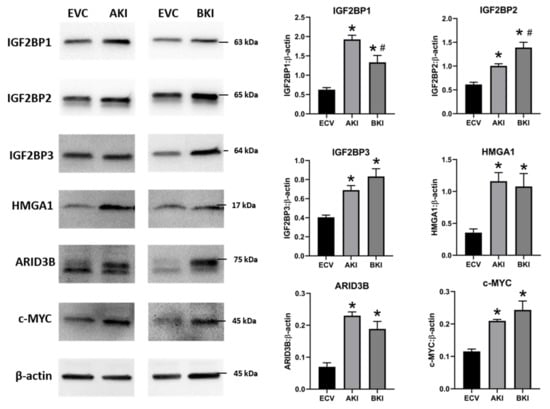
Figure 10. Representative immunoblots for IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, c-MYC, and β-actin, and densitometric analysis of immunoblotting results in AKI and BKI iOTR cells compared to EVC (n = 3), where * p < 0.05 vs. EVC and # p < 0.05 vs AKI.
4. Overexpression of LIN28 Led to Significant Increase in Trophoblast Cell Proliferation
The role of LIN28-let-7 miRNA axis on the functionality of iOTR cells was determined by measuring proliferation of AKI and BKI iOTR cells compared to EVC after 4 h, 24 h, 48 h, and 72 h. The results showed that proliferation of both AKI and BKI iOTR cells was significantly increased at 24 h, 48 h, and 72 h compared to EVC (Figure 11A). Furthermore, proliferation of BKI iOTR cells was not different at 24 h but was significantly higher at 48 h and 72 h compared to AKI iOTR cells (Figure 11A). The matrigel invasion assay showed that there was no significant change in the invasion index of AKI and BKI iOTR cells compared to EVC (Figure 11B). These results suggest that increased proliferation of AKI and BKI iOTR cells is due to increased expression of proliferation-associated genes in these cells compared to EVC.
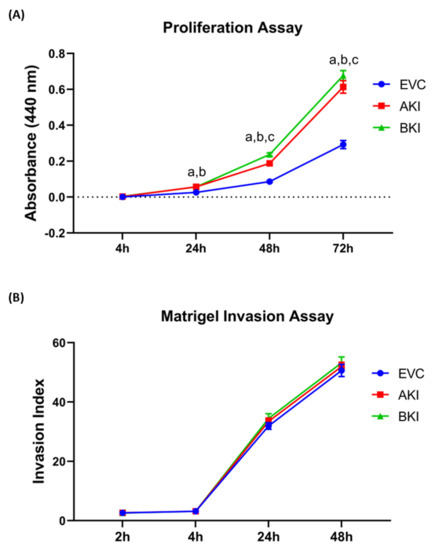
Figure 11. (A) Proliferation of AKI, BKI, and EVC iOTR cells (n = 4/treatment) was measured after 4 h, 24 h, 48 h, and 72 h using Quick Cell Proliferation Assay Kit. (B) Invasion of AKI, BKI, and EVC iOTR cells (n = 4) measured after 2 h, 4 h, 24 h, and 48 h using the Matrigel Invasion Assay Kit; where a, p < 0.05 for AKI vs. EVC; b, p < 0.05 for BKI vs. EVC; and c, p < 0.05 for BKI vs. AKI.
5. Discussion
The pluripotency factors LIN28A and LIN28B inhibit the maturation of let-7 miRNAs [1][2]. Recently, we showed that both Lin28A and LIN28B were significantly decreased and levels of let-7 miRNAs (let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, let-7i) were significantly increased in term human placentas from IUGR pregnancies compared to control pregnancies [3]. We further demonstrated that double knockout of LIN28A and LIN28B in immortalized first trimester human trophoblast (ACH-3P) cells resulted in more robust increase in let-7 miRNAs compared to knockout of either LIN28A or LIN28B. Similarly, double knockin of LIN28A and LIN28B in Sw.71 cells led to more robust decrease in let-7 miRNAs compared to knockin of either LIN28A or LIN28B [3]. In this study, we show that RNA interference (RNAi) of LIN28A or LIN28B in sheep TE in vivo resulted in significant increase in let-7 miRNAs (let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, let-7i). Moreover, the conceptus elongation was significantly reduced after RNAi of LIN28A or LIN28B in TE.
Although animal models with global gene knockout or knockdown have been extensively and successfully used in many studies, it is difficult to exclude the effect of global gene manipulation while focusing on mechanisms involved in one tissue type or organ. Incubating the day 9-hatched sheep blastocyst with shRNA expressing lentiviral particles caused viral infection of only trophoblast cells while all other cells including the inner cell mass were spared of lentiviral infection [4]. Hence, significant reduction in LIN28A or LIN28B in day 16 TE conceptus was restricted to the trophoblast cells only. Conceptus elongation in sheep was due to rapid proliferation of trophoblast cells [5][6][7]; therefore, a significant reduction in conceptus length at day 16 after trophectoderm-specific LIN28A or LIN28B knockdown indicates reduced proliferation of trophoblast cells.
LIN28 is a part of a complex genetic pathway known to regulate multiple downstream targets [8]. Inhibition of biogenesis of mature let-7 miRNAs is one of the main pathways through which LIN28 regulates the expression of its downstream targets [9][10]. Let-7 miRNAs reduce expression of many genes by degrading their mRNAs or inhibiting translation [1]. Our results show that RNAi of LIN28A or LIN28B and resultant increase in let-7 miRNAs in day 16 TE significantly reduced IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC. Previous studies have shown the role of IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC in cell proliferation [1][11][12][13][14][15][16][17][18], suggesting that reduced proliferation of trophoblast cells after LIN28A or LIN28B knockdown in TE is due to reduced expression of proliferation-associated genes (Figure 12).
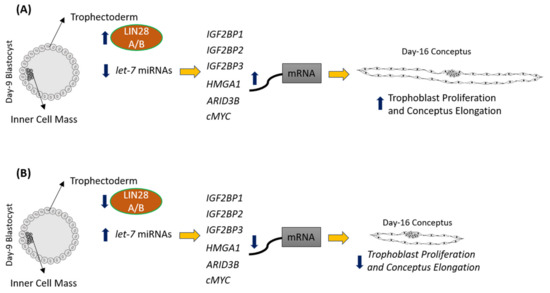
Figure 12. Graphical abstract. (A) In control day 9 TE, let-7 miRNAs are low because of high LIN28A and LIN28B. Because of low levels of let-7 miRNAs, IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC are higher leading to increased proliferation of trophoblast cells and hence conceptus elongation. (B) In LIN28A or LIN28B KD day 9 TE, let-7 miRNAs are higher. Elevated let-7 miRNAs target IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC, leading to reduced expression of these genes, leading to reduced proliferation of trophoblast cells and, hence, reduced conceptus elongation.
To further investigate the role of the LIN28-let-7 miRNA axis in sheep trophoblast cells in vitro, OTR cells were generated from day 16 TE. Surprisingly, both LIN28A and LIN28B were depleted and the let-7 miRNAs were significantly higher in OTR cells compared to day 16 TE. The senescence of OTR cells after only a few passages along with high let-7 miRNAs suggests that these cells are a differentiated phenotype of trophoblast cells compared to day 16 TE. Furthermore, the expression of proliferation-associated genes including IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC was also significantly reduced in OTR cells compared to day 16 TE. To overcome the OTR cell senescence, we generated immortalized ovine trophoblast (iOTR) cells by expressing hTERT in these cells. The OTR cells were differentiated and depleted LIN28 and immortalization with hTERT expression did not change LIN28 expression in iOTR cells. Immortalized human first trimester trophoblast cells (Sw.71 cells) were also generated by expressing hTERT in first trimester human trophoblast cells at passage 3 [19]. Sw.71 cells also had depleted LIN28A and LIN28B and high let-7 miRNAs compared to other trophoblast-derived cell lines such as ACH-3P cells [3]. The iOTR cells generated in this study were similar to Sw.71 cells in terms of LIN28 and let-7 miRNAs expression, which may be because both cell lines were generated by immortalizing the passaged trophoblast cells. The contrasting levels of LIN28, let-7 miRNAs, and let-7 miRNA target genes between day 16 TE and iOTR cells should be taken in consideration if using these cells for further studies.
To generate iOTR cells that were more similar to day 16 TE, we overexpressed LIN28A or LIN28B in iOTR cells. Overexpression of LIN28A or LIN28B in iOTR cells led to a significant decrease in let-7 miRNAs and significant increase in expression of IGF2BP1, IGF2BP2, IGF2BP3, HMGA1, ARID3B, and c-MYC compared to control. The proliferation of both AKI and BKI iOTR cells was significantly increased compared to control; however, there was no change in cell invasion. We recently showed that knockout of LIN28A and LIN28B in ACH-3P cells reduced cell proliferation but did not affect cell invasion [20]. Both ACH-3P and iOTR cells have low invasion; therefore, it would be hard to see a further reduction in invasion. Moreover, cell proliferation and invasion are two distinct and critical processes during placental development. Reduced trophoblast cell proliferation without reduced invasion can be sufficient to cause impaired conceptus elongation and attachment. These results suggest that the LIN28-let-7 miRNA axis plays a role in proliferation of immortalized trophoblast cells by regulating the expression of genes associated with cell proliferation. We suggest that the iOTR cells overexpressing LIN28A and LIN28B would be a better choice to study molecular mechanisms in ovine trophoblast cells compared to iOTR cells with depleted LIN28A and LIN28B.
To our knowledge, this is the first in vivo study defining the role of LIN28-let-7 miRNA axis in early placental development by trophoblast-specific RNAi of LIN28A or LIN28B. Due to a wide range of let-7 miRNAs target genes, as well as the ability of LIN28 to directly bind the mRNA of different genes, LIN28 knockdown might be affecting many different genetic pathways in trophoblast cells, which are yet to be explored. Knockdown of LIN28A or LIN28B and high let-7 miRNAs in TE led to reduced conceptus elongation, which can result in impaired placentation, fetal growth restriction, loss of pregnancy, and reduced fertility in domestic ruminants. We recently showed that term human placentas from IUGR pregnancies have low LIN28 and high let-7 miRNAs [3]. MicroRNAs can be easily measured in tissue biopsies, blood, and other biological samples. Based on our studies, we suggest that low LIN28 or high let-7 miRNAs in placenta could be detected in blood and be used as potential biomarkers for intrauterine growth restriction.
References
- Benjamin Boyerinas; Sun-Mi Park; Noam Shomron; Mads M. Hedegaard; Jeppe Vinther; Jens S. Andersen; Christine Feig; Jinbo Xu; Christopher B. Burge; Marcus E. Peter; Identification of Let-7-Regulated Oncofetal Genes. Cancer Research 2008, 68, 2587-2591, 10.1158/0008-5472.can-08-0264.
- Kimberly M. Jeckel; Alexander C. Boyarko; G J Bouma; Q A Winger; Russell V. Anthony; Chorionic somatomammotropin impacts early fetal growth and placental gene expression. Journal of Endocrinology 2018, 237, 301-310, 10.1530/joe-18-0093.
- Asghar Ali; Russell V. Anthony; Gerrit J. Bouma; Quinton A. Winger; LIN28‐ let‐7 axis regulates genes in immortalized human trophoblast cells by targeting the ARID3B‐complex. The FASEB Journal 2019, 33, 12348-12363, 10.1096/fj.201900718rr.
- Callie M. Baker; Lindsey N. Goetzmann; Jeremy D. Cantlon; Kimberly M. Jeckel; Quinton A. Winger; Russell V. Anthony; Development of ovine chorionic somatomammotropin hormone-deficient pregnancies. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2016, 310, R837-R846, 10.1152/ajpregu.00311.2015.
- L E Rowson; R M Moor; Development of the sheep conceptus during the first fourteen days. Journal of Anatomy 1966, 100, 777-785.
- S Wintenberger-Torrés; J E Fléchon; Ultrastructural evolution of the trophoblast cells of the pre-implantation sheep blastocyst from day 8 to day 18. Journal of Anatomy 1974, 118, 143-153.
- Juhui Wang; Michel Guillomot; Isabelle Hue; Cellular organization of the trophoblastic epithelium in elongating conceptuses of ruminants. Comptes Rendus Biologies 2009, 332, 986-997, 10.1016/j.crvi.2009.09.004.
- J. Tsialikas; J. Romer-Seibert; LIN28: roles and regulation in development and beyond. Development 2015, 142, 2397-2404, 10.1242/dev.117580.
- Hosuk Lee; Sungwook Han; Chang Seob Kwon; Daeyoup Lee; Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell 2016, 7, 100-113, 10.1007/s13238-015-0212-y.
- Mien-Chie Hung; Pai-Sheng Chen; Gunnar Johansson; Min-Liang Kuo; Function and regulation of let-7 family microRNAs.. MicroRNA 2012, 1, 34-39, 10.2174/2211536611201010034.
- Eric Devor; Henry D. Reyes; Nna A. Santillan; Mark Santillan; Chinenye Onukwugha; Michael J. Goodheart; Kimberly K. Leslie; Placenta-Specific Protein 1: A Potential Key to Many Oncofetal-Placental OB/GYN Research Questions. Obstetrics and Gynecology International 2014, 2014, 1-5, 10.1155/2014/678984.
- Valerie B. Sampson; Nancy H. Rong; Jian Han; Qunying Yang; Virginie Aris; Patricia Soteropoulos; Nicholas J. Petrelli; Stephen P. Dunn; Leslie J Krueger; MicroRNA Let-7a Down-regulates MYC and Reverts MYC-Induced Growth in Burkitt Lymphoma Cells. Cancer Research 2007, 67, 9762-9770, 10.1158/0008-5472.can-07-2462.
- Bell, J.L.; Wächter, K.; Mühleck, B.; Pazaitis, N.; Köhn, M.; Lederer, M.; Hüttelmaier, S; Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): Post-transcriptional drivers of cancer progression. Cell. Mol. Life Sci. 2013, 70, 2657–2675.
- Wei Zhao; Dan Lu; Liang Liu; Juan Cai; Yu Zhou; Ying Yang; Yu Zhang; Jun Zhang; Insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3) promotes lung tumorigenesis via attenuating p53 stability. Oncotarget 2017, 8, 93672-93687, 10.18632/oncotarget.21280.
- Lily Mahapatra; Neal Andruska; Chengjian Mao; Jeremy Le; David J. Shapiro; A Novel IMP1 Inhibitor, BTYNB, Targets c-Myc and Inhibits Melanoma and Ovarian Cancer Cell Proliferation. Translational Oncology 2017, 10, 818-827, 10.1016/j.tranon.2017.07.008.
- Yanchun Zhou; Xiuhua Meng; Shaoying Chen; Wei Li; Delin Li; Robert H. Singer; Wei Gu; IMP1 regulates UCA1-mediated cell invasion through facilitating UCA1 decay and decreasing the sponge effect of UCA1 for miR-122-5p. Breast Cancer Research 2018, 20, 32, 10.1186/s13058-018-0959-1.
- Marcell Lederer; Nadine Bley; Christian Schleifer; Stefan Hüttelmaier; The role of the oncofetal IGF2 mRNA-binding protein 3 (IGF2BP3) in cancer. Seminars in Cancer Biology 2014, 29, 3-12, 10.1016/j.semcancer.2014.07.006.
- Cao, J.; Mu, Q.; Huang, H. The Roles of Insulin-Like Growth Factor 2 mRNA-Binding Protein 2 in Cancer and Cancer Stem Cells. Available online: https://www.hindawi.com/journals/sci/2018/4217259/ (accessed on 18 November 2019).
- Shawn Chavez; Vikki M. Abrahams; Ayesha B. Alvero; Paulomi B. Aldo; Yuehong Ma; Seth Guller; Roberto Romero; Gil Mor; The Isolation and Characterization of a Novel Telomerase Immortalized First Trimester Trophoblast Cell Line, Swan 71. Placenta 2009, 30, 939-48, 10.1016/j.placenta.2009.08.007.
- Rachel West; E S McWhorter; A Ali; L N Goetzman; Jennifer Russ; C L Gonzalez-Berrios; R V Anthony; G J Bouma; Q A Winger; HMGA2 is regulated by LIN28 and BRCA1 in human placental cells†. Biology of Reproduction 2018, 100, 227-238, 10.1093/biolre/ioy183.
