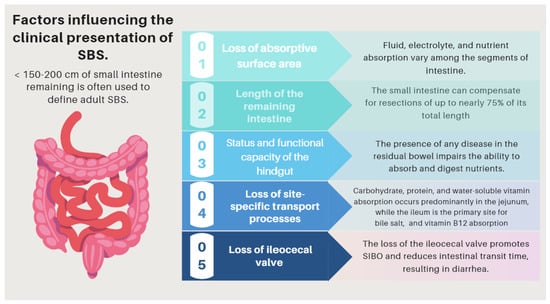
Short bowel syndrome (SBS) is a malabsorptive condition that is most often caused by a massive resection of the small intestine [52]. Its prevalence is 3–4 per million [53] and occurs in about 15% of adult patients undergoing an intestinal resection, either massive (3/4) or from multiple sequential resections (1/4) [54]. Although its causes may be diverse, in the present manuscript, we will refer to the one that results from a massive bowel resection [55]. SBS and intestinal failure (IF) are not necessarily synonymous. Intestinal failure describes the state when an individual’s gastrointestinal function is inadequate to maintain his or her nutrient and hydration status without intravenous or enteral supplementation [56,57,58].
- malnutrition
- metabolism
- micronutrients
- nutritional deficiencies
- dietitian
1. Clinical Presentation
1.1. Global Outlook
| Complications Related to SBS | Pathogenesis |
|---|---|
| (I) Complications related to SBS pathophysiology and its underlying pathology | The pattern of nutrient absorption native to the parts of the gastrointestinal tract is shown in Figure 1. |
| Peptic ulcer | Hypergastrinemia resulting from a failure of enterogastrone release (e.g., VIP, GIP, neurotensin, peptide YY, and GLP-1). Treatment with antisecretory drugs could also aggravate SIBO due to hipoclorhydria [16][73]. |
| Electrolyte disturbances: hypocalcemia, hypokalemia, and hypomagnesemia |
Occur especially when large-volume diarrhea is present. (e.g., associated with an end jejunostomy). |
| D-lactic acidosis (D-LA) | The SBS microbiota, since it is rich in Lactobacillus, leads to the accumulation of fecal lactate. Lactate does not accumulate in healthy human feces because it is absorbed by intestinal cells, but in some SBS patients, the high amount of lactate found in feces indicates that production exceeds absorption capacities by the host. Excess lactate released into the colon is fermented by bacteria and converted into D-lactate, which has neuro-toxic effects [10][11][12][67,68,69]. |
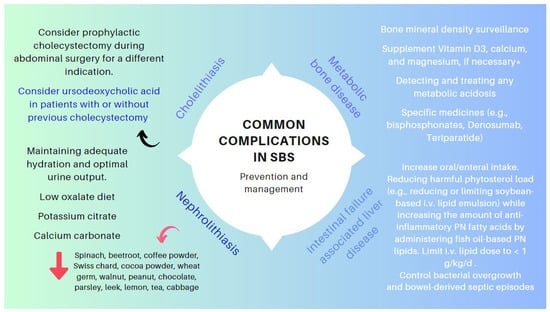
| Cholelithiasis | In the presence of an ileum resection, it breaks the enterohepatic circle of bile salts, causing a reduced biliary excretion and a marked decrease of the bile salt pool in the duodenal lumen. Consequently, cholesterol is oversaturated, favoring the formation of biliary stones [13][14][70,71]. |
| Nephrolithiasis | As a result of steatorrhea, increased free fatty acids are available to bind to calcium, resulting in an increased concentration of non-bound oxalate, which is easily absorbed across the colonic mucosa, where it is moving to the kidneys. Nephrolithiasis is more common among patients with an intact colon. The risk of nephrolithiasis is enhanced by volume depletion, metabolic acidosis, and hypomagnesemia, resulting in decreased renal perfusion, urine output, pH, and citrate excretion [15][16][72,73]. |
| Metabolic osteopathy | Metabolic changes that occur in SBS result in the depletion of calcium, magnesium, and vitamin D, which results in the demineralization of bone. The release of pro-inflammatory cytokines, steroid use, PN, chronic metabolic acidosis, and renal insufficiency may contribute to the development of metabolic osteopathy [17][74]. |
| (II) Complications related with nutritional therapy | Pathogenesis |
| Thrombus-associated venous occlusion | Central venous catheter (CVC)-related thrombosis (CRT) is a severe complication of parenteral nutrition (HPN), which increases its associated morbidity (due to pulmonary embolism) and mortality rates of this population [18][19][75,76]. |
| Catheter-associated central line bloodstream infections | Primary and intravascular catheter-associated bloodstream infections represent an important clinical entity in the intensive care unit (ICU) and has a poor effect on outcomes. Over-abundant levels of Proteobacteria have been found in the feces of patients with SBS presenting with Ca-CLBI [20][21][22][23][77,78,79,80]. |
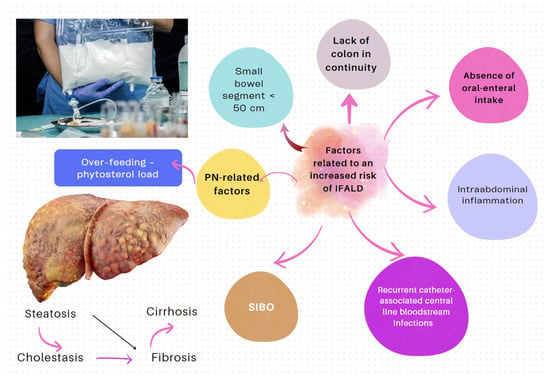
| IF-associated liver disease (IFALD) | IFALD is a possible complication in patients with IF who need intravenous support for survival due to severe intestinal dysfunction. An elevation of aminotransferases or cholestasis enzymes in this setting should raise clinical suspicion of this entity, which may progress from hepatic steatosis to cirrhosis. Some factors that increase the risk of this condition are shown in Figure 6. Liver cholestasis can be a life-threatening complication during HPN and may lead to a combined liver–intestinal transplantation (Figure 6) [22][24][25][26][17,79,81,82]. |
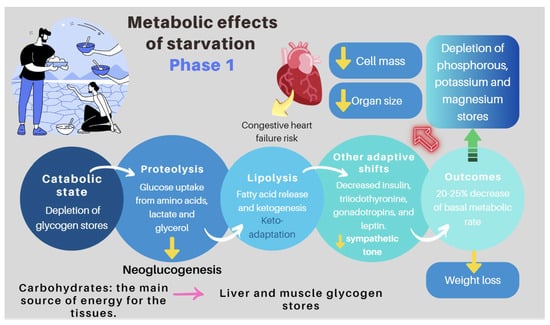
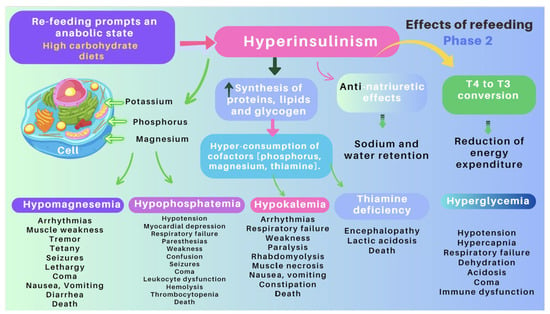
| Re-feeding syndrome (RFS) | The switch from a catabolic state to an anabolic state in malnourished patients undergoing refeeding (orally, enterally, or parenteral) may be the cause of all these clinical manifestations, which, in some cases, can lead to death. RS include a complex and extensive list of changes, such as hypophosphatemia, hypomagnesemia, hypokalemia, hyponatremia, hypocalcemia, hyperglycemia, and vitamin deficiency (especially thiamine deficiency), all of which are accompanied by clinical signs and symptoms, reflecting organ dysfunction (cardiovascular, renal, respiratory, and neurological manifestations, among others). Figure 7 and Figure 8 summarize the relationship between the pathophysiology of RS and its clinical presentation [27][28][29][30][31][32][33][83[,8434,85,86],87,88,89,90]. |
1.2. D-lactic Acidosis
1.3. Refeeding Syndrome (RS)

2. Basic Principles of Nutritional Management
-
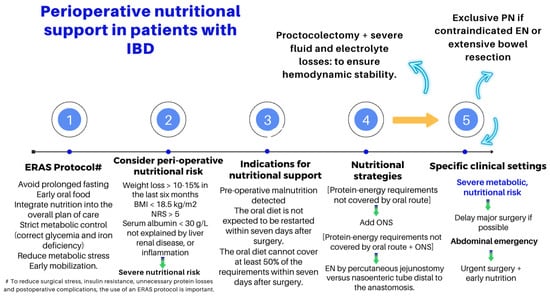
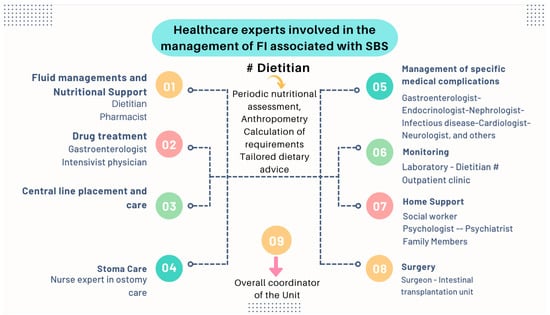
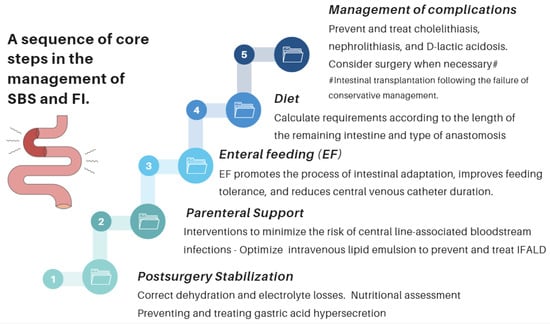
Nutritional interventions to treat SBS include enteral and PN, intestinal rehabilitation techniques to increase the absorptive ability of the residual bowel, and surgical reconstruction designed to enhance the surface area for absorption [37][38][39][92,93,94]. Therefore, managing these patients requires a comprehensive and interdisciplinary approach in centers with proven experience in treating such challenging scenarios [38][39][40][41][42][93,94,95,96,97]. This issue is essential, as has been demonstrated by Geransar P et al., who reported a low level of awareness of chronic IF among non-specialist healthcare professionals [40][95]. Figure 59 highlights the components of a multidisciplinary team caring for these patients in a highly specialized center [38][41][93,96], and Figure 610 outlines the fundamental steps to be followed in the assessment and treatment of these patients [42][
- Many patients with less severe forms of SBS may be fed orally early. The dietary and nutritional management of these patients necessitate to understand the physiology and to consider the individual anatomy and adaptation phase. During the hypersecretory phase, fluid losses are usually the largest. Dehydration and saline depletion can occur during any phase, especially in patients without a colon, and particularly in case of an end jejunostomy [
- 98].
| Requirement |
|---|
- 97].
-
Type 1: end jejunostomy.
| Comment |
|---|
- This is the most unfavorable phenotype, as malabsorption is more severe, and it presents with a high ostomy output. Patients without a colon and <100 cm of the jejunum have a higher risk of requiring long-term PN. Indeed, dehydration, hydroelectolytic abnormalities, acidosis, and renal failure are more likely in these patients [47][57]. Sodium absorption in the jejunum is dependent on water fluxes and is coupled to the absorption of glucose. For this reason, hydration with hypotonic solutions (e.g., water, tea, or coffee) should be discouraged, as they only exacerbate fluid losses through the stoma. Hypertonic drinks (e.g., fruit juices) should also not be recommended as they cause osmotic diarrhea. Some measures that may be useful for these patients are lowering the intake of sugars, decreasing the size of intakes, and take the oral rehydration solutions (ORSs) whose composition is best suited to promote the entry of sodium and water into the enterocytes
| Requirement |
|---|
| Comment |
|---|
- Table 26):
| Energy | 35–45 kcal/kg/day. In some cases, increasing the energy intake up to 60 kcal/kg/day may be necessary. |
Patients with SBS develop compensatory hyperphagia, and it is advisable to take 5–6 meals spaced out during the day. | |
| Carbohydrates | 20–40% of the daily energy target. | In the absence of a colon, it is not possible to rescue energy inherent in the production of short-chain fatty acids from the bacterial fermentation of sugars. | |
| Protein | 1.5–2.0 g/kg/day or 20–30% of the daily energy target. | It is preferable to choose lean proteins of high biological value. | |
| Fat | 40–60% of the daily energy target. | Choose essential fatty acids as the main component of fat intake. Consider MCTs in cases of malabsorption. | |
| Fluids | Reduce oral hypotonic fluids to 500 mL/day #. Separating solids and liquids (i.e., do not drink anything for half an hour before or after a meal). |
Add sodium chloride to any liquid feeds to make the sodium concentration near 100 mmol/L while keeping osmolality near 300 mOsmol/kg *. | |
| Oxalate | The restriction is not necessary. | Calcium oxalate stones only occur in patients with a preserved colon. | |
| Vitamin B6—pyridoxine | |||
| (DRI: 1.3–1.7 mg/day for 14 to over 70 years) | |||
| Microcytic anemia, seborrheic dermatitis with cheilosis and glossitis, angular stomatitis, epileptiform convulsions, confusion, and/or depression | |||
| PLP levels. Red cell PLP in serious patients or in the presence of inflammation | |||
| Oral: 1–2 capsules daily | |||
| (multivitamin: B1, B2, B3 B5, B6, and B7) | |||
| HPN and long-term PN: 4 mg/day | |||
| Vitamin B7—biotin (DRI: 40 μg/day) |
Ataxia, dermatitis, and alopecia | Direct measure of urine and blood biotin that must be completed with the determination of biotin activity | Oral: 1–2 capsules daily (multivitamin: B1, B2, B3 B5, B6, and B7) |
| HPN and long-term PN: 60 μg/day | |||
| Vitamin B9—folic acid (DRI: 330 μg/day DFE) |
Glossitis, megaloblastic anemia, pancytopenia, oral ulcers, angular stomatitis, and neuropsychiatric manifestations | Folate level in the plasma or serum—short-term status. In RBCs—long-term status | Oral: 1 mg daily |
| HPN and long-term PN: 400 μg/day | |||
| Vitamin B12—cobalamin (DRI: 2.4 μg/day) |
Hematological, neurological, neuropsychiatric, and cognitive symptoms | Combination of at least two bio- markers (e.g., holo-TC and MMA), with serum cobalamin as a replacement for holo-TC when the measurement of this latter is unavailable | Oral: 1–2 capsules daily (multivitamin: B1, B2, B3 B5, B6, and B7) |
| HPN and long-term PN: 5 μg/day | |||
| Vitamin C—ascorbic acid (DRI: 90–100 mg/day) |
Lassitude; shortness of breath; anemia; poor wound healing; myalgia and bone pain; loose teeth; spongy and purplish gums that are prone to bleeding; bulging eyes; scaly, dry, and brownish skin; dry hair that breaks; edema; petechiae; and easy bruising | Total plasma vitamin C (sum of AA and DHAA) or AA. | Oral: 200–500 mg daily ǂ |
| HPN and long-term PN: 100–200 mg/day | |||
| Fat-soluble vitamins | Doses (all values per day) | ||
| Vitamin A (DRI: 700–900 µg/day) |
Night blindness, Bitot spots, foamy appearance on the conjunctiva, xerophthalmia, increased susceptibility to infections, and impairment of the intestinal immune and barrier function | Serum retinol | Oral: 5000–50,000 IU daily # |
| HPN and long-term PN: 800–1100 µg/day | |||
| Vitamin D (DRI: 15–20 µg/day) |
Osteomalacia and nutritional rickets; increased susceptibility to infections | Serum 25-hydroxyvitamin D (25(OH)D) |
<12 ng/mL: oral: 50,000 IU# once weekly (or calcitriol 0.25–2 mg daily), followed by maintenance:
|
| HPN and long-term PN: 200 IU/5 µg/day | |||
| Vitamin E (DRI: 15 mg/day) |
Neurological symptoms (balance and coordination disorders and peripheral neuropathy) and muscle weakness | Serum alpha-tocopherol | Oral: 400 IU up to 3 times daily |
| HPN and long-term PN: 9–10 mg/day | |||
| Vitamin K (DRI: 90–120 µg/day) |
Prolongation of prothrombin time with impaired clotting or bleeding, poor bone development, osteoporosis, and increased cardiovascular disease | Combination of biomarkers and dietary intake | Oral: 2.5 to 10 mg twice weekly to daily, or 10-mg single dose #; can be repeated 48–72 h later |
| HPN & long-term PN: 150 µg/day, usually provided by lipid emulsions | |||
| Trace mineral | Doses (all values per day) | ||
| Iron (DRI: 8 mg/day. 18 mg/day for female 19–50 years old) |
Microcytic anemia | Serum ferritin, sideremia, and transferrin saturation (%) | Oral: 100–200 mg once daily or every other day # ǂ |
| HPN and long-term PN: 1.1 mg/day | |||
| Copper (DRI: 1.1–2 mg/day) |
Microcytic anemia, neutropenia, osteoporosis, hair depigmentation, cardiac arrhythmias, delayed wound healing, and myeloneuropathy | Serum copper | Oral: 2 mg of elemental copper daily ǂ Higher doses may be needed |
| HPN and long-term PN: 0.3–0.5 mg/day- | |||
| Chromium (DRI: 20–35 µg/day) |
Hyperglycemia, insulin resistance, elevated plasma fatty acids, weight loss, and peripheral neuropathy | Serum chromium | Oral: 100–200 mg up to 3 times daily |
| HPN and long-term PN: 10–15 µg/day | |||
| Zinc (DRI: 8–11 mg/day) |
Impairment of immune defense, reduced growth rate, alopecia, skin rash of the face, groins, hands, and feet, delayed sexual development and bone maturation, impaired wound healing, diarrhea, and blunting of taste and smell | Serum zinc | Oral: 50 mg elemental zinc (once or twice daily) Dietary sources such as oysters and mussels can also be considered |
| HPN and long-term PN: 3–5 mg/day | |||
| Selenium (DRI: 55 µg/day) |
Cardiomyopathy, skeletal muscle myopathy, and skin and nail impact | Serum selenium | Oral: 100–200 mg daily |
| HPN and long-term PN: 60–100 µg/day |
-
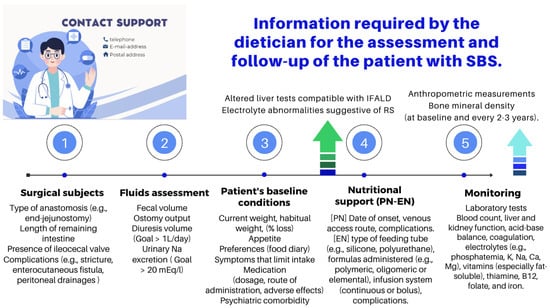
The role of an experienced dietitian is also fundamental in the initial assessment and monitoring of the nutritional status of patients with SBS. Concerning this point, the dietitian should obtain information regarding multiple details related to the type of surgery performed, the patient’s baseline post-operative conditions (renal function, water–electrolyte, and acid–base balance), their degree of malnutrition, and the type of nutritional support received (enteral or parenteral), including access routes, as well as their associated complications (Figure 6 and Figure 7).
- [44][45][46]. Although few patients with severe SBS can discontinue PN before hospital discharge, more than 50% of adults will be able to wean completely from PN within five years of their diagnosis. PN volume can be decreased when the patient begins to tolerate oral nutrition. This is possible if the volume or flow of feces from the rectum or through the ostomy is adequately reduced and the patient begins to gain weight. Enteral nutrition (EN) provides clear benefits, prevents villous atrophy, helps preserve the intestinal epithelial barrier, enhances the local immunity needed to avoid SIBO, and promotes the mechanisms of bowel adaptation, enhancing weaning from parenteral nutrition. The introduction of EN should always be prudent and judicious [45][46]. One approach is to start EN by providing 5% of the total calories and increasing this ratio every 3–7 days and assess tolerance. Patients who require long-term PN cannot be kept in hospital indefinitely, and thus transition to home PN when they are clinically stable. To maximize patient mobility and convenience at home, PN infusion time is minimized, and the solution is infused overnight. PN infusion time can typically be reduced (cycled) to 10–15 h, depending on patient tolerance [41][42]. Notably, the SBS patient receiving home PN is still at risk of micronutrient deficiencies, as well as liver and bone complications, and requires regular monitoring and supplementation with PN (Table 2).
-
Many patients with less severe forms of SBS may be fed orally early. The dietary and nutritional management of these patients necessitate to understand the physiology and to consider the individual anatomy and adaptation phase. During the hypersecretory phase, fluid losses are usually the largest. Dehydration and saline depletion can occur during any phase, especially in patients without a colon, and particularly in case of an end jejunostomy [43].
-
The role of an experienced dietitian is also fundamental in the initial assessment and monitoring of the nutritional status of patients with SBS. Concerning this point, the dietitian should obtain information regarding multiple details related to the type of surgery performed, the patient’s baseline post-operative conditions (renal function, water–electrolyte, and acid–base balance), their degree of malnutrition, and the type of nutritional support received (enteral or parenteral), including access routes, as well as their associated complications (Figure 10 and Figure 11).
-
Almost all patients with SBS need PN during the early period after a resection. PN should be initiated and adjusted to meet the patient’s fluid, electrolyte, energy, protein, and micronutrient needs. The literature provides excellent reviews of PN performance in this setting [43]
-
Almost all patients with SBS need PN during the early period after a resection. PN should be initiated and adjusted to meet the patient’s fluid, electrolyte, energy, protein, and micronutrient needs. The literature provides excellent reviews of PN performance in this setting [98,99,100,101]. Although few patients with severe SBS can discontinue PN before hospital discharge, more than 50% of adults will be able to wean completely from PN within five years of their diagnosis. PN volume can be decreased when the patient begins to tolerate oral nutrition. This is possible if the volume or flow of feces from the rectum or through the ostomy is adequately reduced and the patient begins to gain weight. Enteral nutrition (EN) provides clear benefits, prevents villous atrophy, helps preserve the intestinal epithelial barrier, enhances the local immunity needed to avoid SIBO, and promotes the mechanisms of bowel adaptation, enhancing weaning from parenteral nutrition. The introduction of EN should always be prudent and judicious [100,101]. One approach is to start EN by providing 5% of the total calories and increasing this ratio every 3–7 days and assess tolerance. Patients who require long-term PN cannot be kept in hospital indefinitely, and thus transition to home PN when they are clinically stable. To maximize patient mobility and convenience at home, PN infusion time is minimized, and the solution is infused overnight. PN infusion time can typically be reduced (cycled) to 10–15 h, depending on patient tolerance [96,97]. Notably, the SBS patient receiving home PN is still at risk of micronutrient deficiencies, as well as liver and bone complications, and requires regular monitoring and supplementation with PN (Table 6).
3. Recommendations of Scientific Societies
| Energy | 35–45 kcal/kg/day. In some cases, increasing the energy intake up to 60 kcal/kg/day may be necessary. |
Patients with SBS develop compensatory hyperphagia, and it is advisable to take 5–6 meals spaced out during the day. |
| Carbohydrates | 50–60% of the daily energy target. | The colon provides energy (up to 1000 kcal/day) in SBS by releasing the SCFAs resulting from the fermentation of carbohydrates. In addition, it provides nutrition to the colonocytes. |
| Protein | ||
| In SBS, the colon plays a vital role in fluid and electrolyte reabsorption, given the additional fluid that enters the colon with a capacity to absorb up to 6 L daily. | ||
| Fat | 20–30% of daily energy target. | In jejunum–colon patients, unabsorbed long-chain fatty acids in the colon are likely to reduce the transit time and reduce their water and sodium absorption, making their diarrhea worsen. |
-
Type 2: jejunocolic. It retains a portion of the jejunum anastomosed to a portion of the colon. In these patients, the clinical picture is dominated by diarrhea due to severe malabsorption, vitamin–mineral deficiencies, and subsequent malnutrition. Patients with jejunocolic anastomosis and <50 cm of the jejunum also have a higher risk of requiring long-term PN. The nutritional recommendations for those who recover intestinal autonomy are as follows (Table 3
| Type of Micronutrient and Average Nutritional Intake Ranges | Clinical Signs Reflecting a Deficiency | Measurement | Typical Supplementation in SBS * |
|---|---|---|---|
| Water-soluble vitamins | Doses (all values per day) | ||
| Vitamin B1—thiamine (DRI: 1.1–1.2 mg/day) |
Mental changes (apathy, decrease in short-term memory, confusion, and irritability), cognitive deficits, congestive heart failure, and metabolic lactic acidosis | Whole-blood ThDP or RBCs | Oral: 1–2 capsules daily (multivitamin: B1, B2, B3 B5, B6, and B7) |
| HPN and long-term PN: 2.5 mg/day | |||
| Vitamin B2—riboflavin (DRI: 1.3 mg/day—males. 1.1 mg/day—females) |
Seborrheic dermatitis of the face, trunk, and scrotum, oral buccal lesions, ocular manifestations, marrow aplasia, and normochromic, normocytic anemia | Glutathione reductase activity in red blood cells | Oral: 1–2 capsules daily (multivitamin: B1, B2, B3 B5, B6, and B7) |
| HPN and long-term PN: 3.6 mg/day | Consider MCTs only in the case of severe malabsorption. MCT does not contain essential fatty acids. |
-
Type 2: jejunocolic. It retains a portion of the jejunum anastomosed to a portion of the colon. In these patients, the clinical picture is dominated by diarrhea due to severe malabsorption, vitamin–mineral deficiencies, and subsequent malnutrition. Patients with jejunocolic anastomosis and <50 cm of the jejunum also have a higher risk of requiring long-term PN. The nutritional recommendations for those who recover intestinal autonomy are as follows (Table 7):
| 1.5–2.0 g/kg/day or 20–30% of the daily energy target. | ||
| It is preferable to choose lean proteins of high biological value. | ||
| Fluids | Isotonic/hypotonic #. | |
| Oxalate | The diet should be low in oxalate. | Nephrolithiasis only occurs in patients with a preserved large bowel. |
- ):
-
Type 3: jejunoileocolic. These patients retain their entire colon and ileocecal valve along with a portion of their terminal ileum and jejunum. This is indeed the most advantageous phenotype, and these patients often do not require additional nutritional support because the ileum has a greater ability to adapt. This subgroup does not usually develop malnutrition, electrolyte disorders, or dehydration [47].
-
Type 3: jejunoileocolic. These patients retain their entire colon and ileocecal valve along with a portion of their terminal ileum and jejunum. This is indeed the most advantageous phenotype, and these patients often do not require additional nutritional support because the ileum has a greater ability to adapt. This subgroup does not usually develop malnutrition, electrolyte disorders, or dehydration [57].
4. Common Recommendations for All Three Phenotypes
5. Vitamin and Mineral Replacement
| Vitamin B3—niacin | |||
| (DRI: 16 mg/day—adolescents and adult males > 14 years. 14 mg/day—females > 14 years) | |||
| Dementia, dermatitis, and diarrhea | Blood or tissue NAD | Oral: 1–2 capsules daily | (multivitamin: B1, B2, B3 B5, B6, and B7) |
| HPN and long-term PN: 40 mg/day | |||
| Vitamin B5—pantothenic acid (DRI: 5 mg/day for 14 to over 70 years) |
Fall in the diastolic and lability of systolic blood pressure, with postural hypotension, vertigo, and tachycardia. Gastrointestinal and neurological symptoms | Blood pantothenic acid | Oral: 1–2 capsules daily (multivitamin: B1, B2, B3 B5, B6, and B7) |
| HPN and long-term PN: 10 mg/day | |||
6. Pharmacological Treatment
7. Management of Other Specific Conditions
8. Surgical Management
- (1)
-
Preserving the existing intestine: It is common that after the initial resection, some patients need to be re-operated for various reasons (e.g., stenosis and perforations). In these scenarios, avoiding a resection and preserving the existing length of the intestinal remnant (e.g., serosal patching for certain strictures and chronic perforations) are essential. When carrying out a resection becomes unavoidable, an end-to-end anastomosis is preferred to prevent blind loops and, thus, optimize the functionality of the hindgut [101][58].
- (2)
-
Restoration of intestinal continuity, elimination of a stoma with the aim of improving the patient’s quality of life and avoiding some of the complications associated with central venous catheters [47][57].
- (3)
-
Tapering surgery when the remaining small bowel remains excessively dilated [102][153]. Intestinal tapering may be necessary in this context as a dilated intestine increases the risk of mucosal injury, bloodstream infections, and liver disease in patients with SBS [103][154]. Several techniques have been described to taper the dilated small bowel, including longitudinal intestinal lengthening and tapering, serial transverse enteroplasty, and spiral intestinal lengthening and tailoring [102][104][153,155].
- (4)
-
Correction of stenoses, if possible, with stricturoplasties and with remodeling or intestinal plication if needed [105][156].
- (5)
-
Serosal patching for chronic fistulae to prevent avoidable intestinal excisions [106][157].
- (6)
-
Autologous gastrointestinal reconstruction operation: The aim of this procedure is to either enhance the mucosal surface area for absorption (e.g., lengthening procedures) or to slow intestinal transit to facilitate the assimilation of the nutrients or counterbalance stasis that cause gastrointestinal symptoms due to SIBO (e.g., reversing the segments of the intestine) [107][108][158,159], creating intestinal valves, or interposing a colonic segment in the mall intestinal remnant in either an isoperistaltic or antiperistaltic fashion [109][110][111][112][7,160,161,162]. These procedures should only be used in carefully selected patients and in centers with proven experience [113][114][115][163,164,165].
9. Intestinal Transplantation (ITx)
| Clinical Condition | Criteria | Comments |
|---|---|---|
| IAFLD | Forthcoming (total bilirubin above 3–6 mg/dL (54–108 μmol/L), progressive thrombocytopenia, and progressive splenomegaly) or overt liver failure (portal hypertension, hepatosplenomegaly, hepatic fibrosis, or cirrhosis because of IFALD). |
Liver biopsy is the gold standard test to identify the stage of liver disease, the timing of transplantation, and the type of transplant required (isolated ITx or combined liver–ITx) It has been suggested that patients with METAVIR stage II fibrosis (perisinusoidal and portal/periportal fibrosis) should be considered for an isolated ITx, whereas those with stage III (bringing fibrosis) or IV (cirrhosis) should be considered for LITx. |
| Esophageal varices, ascites, and impaired synthetic function are not always present. | ||
| Central venous catheter-related thrombosis (CRVT) | Thrombosis of two or more central veins (loss of right and left internal jugular vein, right and left subclavian vein, or right and left femoral vein). |
CRVT is a severe complication that is responsible for the loss of central venous accesses in patients on HPN and may be an indication for ITx if it affects two or more of the central venous vessels. For adults, this criterion is on a case-by-case basis. |
| Catheter-related bloodstream infection (CRBSI). | Frequent central line sepsis: two or more episodes per year of systemic sepsis secondary to line infections requiring hospitalization; a single episode of line-related fungemia; septic shock and/or acute respiratory distress syndrome. |
Children: two admissions to an intensive care unit because of cardiorespiratory failure (mechanical ventilation or inotrope infusion) due to sepsis. For adults, this criterion is on a case-by-case basis, because recurrent episodes of CRBSI have been demonstrated to not be associated with an increased risk of death. |
| Other indications | Refractory electrolyte changes and frequent episodes of dehydration. High risk of death attributable to underlying diseases, such as congenital mucosal disorders, ultra-short bowel syndrome (gastrostomy; duodenostomy; residual small bowel <10 cm in infants and <20 cm in adults), and invasive intra-abdominal desmoid tumors; patients with high morbidity (frequent hospitalization, narcotic dependency, and inability to function (i.e., pseudo-obstruction; high output stoma)) or a low acceptance of long-term PN, especially in young patients. |
|