Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ludmila Müller and Version 2 by Sirius Huang.
Aging induces numerous physiological alterations, with immunosenescence emerging as a pivotal factor. This phenomenon has attracted both researchers and clinicians, prompting profound questions about its implications for health and disease. Among the contributing factors, one intriguing actor in this complex interplay is human cytomegalovirus (CMV), a member of the herpesvirus family. Latent CMV infection exerts a profound influence on the aging immune system, potentially contributing to age-related diseases.
- aging
- immunosenescence
- cytomegalovirus
- inflammaging
- CMV latency
1. Introduction
Aging is an inevitable part of the human experience, a journey marked by the passage of time that brings with it profound changes across various aspects of life. Among the myriad of alterations that accompany the process of growing older, the impact on the immune system stands out as a particularly significant and multifaceted phenomenon [1][2][3][1,2,3]. Immunosenescence, the gradual and complex shift in immune function associated with aging [1][4][5][6][7][1,4,5,6,7], has emerged as a critical area of scientific investigation, raising crucial questions about its implications for health, disease, and longevity.
The immune system, a remarkable defense network of tissues, cells, and molecules, plays a crucial role in protecting the body from pathogens, such as bacteria, viruses, and other potential threats. Throughout life, it adapts to new challenges, learns to recognize invaders, and forms immunological memories. However, as individuals navigate the journey of aging, this once-vigilant guardian undergoes a transformation [1][8][1,8], rendering the host more vulnerable to infections, impairing its ability to respond effectively to novel threats [6][9][6,9], and exacerbating the risk of various age-associated diseases, including cancer, cardiovascular conditions, and neurodegenerative disorders.
A remarkable feature of immunosenescence is its dynamic interplay with cytomegalovirus, a ubiquitous human pathogen [10][11][12][10,11,12]. CMV, a member of the herpesvirus family, has the remarkable capacity to establish lifelong persistence in the human host, often remaining asymptomatic. Although this virus coexists with the host for decades, its presence has been linked to profound changes in the immune landscape. Over the years, researchers have worked to investigate the role of CMV in shaping immune responses and raising questions about the virus’s contributions to both the aging process and age-related diseases [13][14][15][16][17][18][13,14,15,16,17,18].
2. Unraveling Immunosenescence: Key Concepts and Hallmarks
Aging is a multifaceted process involving the gradual remodeling of various physiological systems, and the immune system is no exception to these age-related alterations [1][4][19][20][21][22][1,4,19,25,26,27]. This phenomenon, termed immunosenescence, is a prominent feature of aging characterized by the progressive changes in immune function, impacting both innate and adaptive immune responses [23][28]. The key concepts and hallmarks briefly outlined below represent a selection of central elements (Figure 1) contributing to the complex phenomenon of immunosenescence:
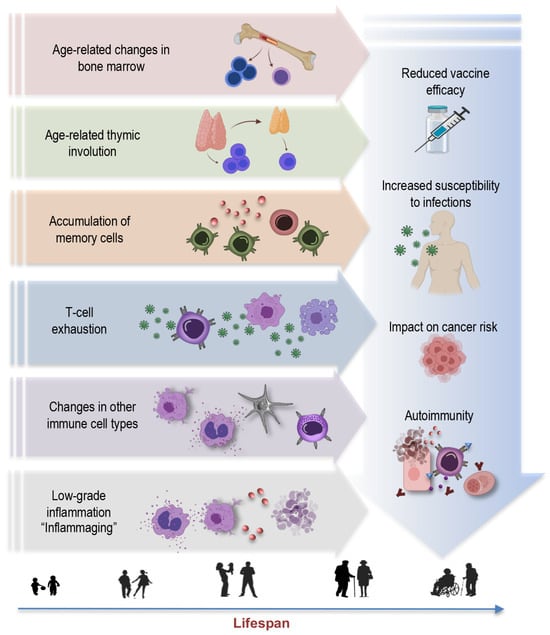
Figure 1.
Key concepts and hallmarks of immunosenescence.
Age-related changes in bone marrow: Aging leads to changes in the bone marrow [21][24][25][26,29,30], a vital component of the immune system. These changes include alterations in hematopoiesis, the process of blood cell formation. The bone marrow produces fewer immune precursor cells, impacting the generation of various immune cells, such as lymphocytes and monocytes. This bone marrow shift further contributes to the overall decline in immune function associated with immunosenescence [26][31]. Understanding these age-related changes in the bone marrow is integral to comprehending the broader concept of immunosenescence.
Thymus involution: Another key concept is thymus involution, characterized by the gradual degeneration of the thymus, a primary lymphoid organ central to T-cell development (Figure 1). This process initiates early in life but becomes more pronounced with aging, leading to a significant decline in thymic function [3][7][27][28][29][3,7,32,33,34]. This results in a diminished output of new T cells and a skewed T-cell repertoire. The effects of age-related thymic involution are profound, as it directly affects the diversity and functionality of the immune system [27][32]. The decreased production of naïve T cells compromises the ability to respond effectively to new pathogens and antigens, contributing to an increased susceptibility to infections and a decline in immune surveillance against tumor cells.
Accumulation of memory cells: An important hallmark feature of immunosenescence is the accumulation of memory T cells and B cells within the aging immune system [5][7][9][11][24][29][30][31][5,7,9,11,29,34,35,36]. These memory cells, formed in response to previous encounters with pathogens, are crucial for mounting rapid and effective immune responses upon re-exposure. However, with advancing age, there is a notable shift in the composition of the immune cell repertoire. The proportion of memory T cells increases, often at the expense of naïve cells, altering the balance within the immune system. Despite an initial increase in memory cells during early life, these cells also undergo senescent changes later in life, with hallmarks such as the loss of CD28 and the accumulation of highly differentiated effector memory T cells [3][32][33][3,37,38]. This skew towards memory cells may compromise the ability to respond to novel pathogens and antigens, impairing the adaptability and robustness of immune defenses in older individuals.
T-cell exhaustion: T-cell exhaustion is a key concept within the realm of immunosenescence [34][39]. This phenomenon refers to a state where T cells lose their functional capabilities, particularly in the context of chronic infections or prolonged exposure to antigens. T-cell exhaustion is characterized by reduced proliferative potential, diminished cytotoxicity, impaired cytokine secretion, and heightened expression of inhibitory receptors, such as programmed cell death protein 1 (PD-1), killer cell lectin-like receptor G1 (KLRG1), and CD57. In the context of aging and chronic viral infections like CMV, the expansion of exhausted T cells, particularly CD28- T cells, is a hallmark of immunosenescence [35][40]. These cells may retain some cytotoxicity and ability to produce Th1 cytokines, but their overall functional decline contributes to weakened immune responses. Understanding T-cell exhaustion is crucial, as it has implications not only for immunosenescence, but also for diseases associated with chronic infections and aging.
Changes in other immune cell types: Immunosenescence extends beyond T cells, affecting various immune cell populations [36][41]. For instance, natural killer (NK) cells, crucial for the defense against infected or malignant cells, also experience alterations with age. The functional decline of NK cells may result in reduced surveillance against transformed or infected cells [4][6][36][37][38][4,6,41,42,43], potentially contributing to higher cancer susceptibility in older individuals. Myeloid cells, including macrophages and dendritic cells, can become more prone to pro-inflammatory activation, leading to the elevated production of pro-inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β). This shift in their functional profile contributes to the low-grade chronic inflammation associated with aging. This pro-inflammatory state also has implications for the functioning of other immune cells, potentially disrupting the immune response to infections and vaccinations [6][36][39][40][6,41,44,45].
Inflammaging: Inflammaging is a central concept in the realm of immunosenescence, representing the chronic, low-grade inflammation that characterizes the aging immune system [20][41][25,46]. This persistent inflammatory background, often driven by factors such as cellular stress, senescent cells, and the secretion of proinflammatory molecules, plays a pivotal role in the pathogenesis of various age-associated conditions, including cardiovascular diseases, cancer, and neurodegenerative disorders [7][20][41][42][7,25,46,47]. Understanding the concept of inflammaging is crucial in elucidating the multifaceted landscape of immunosenescence, as it underscores the intricate interplay between immune aging and age-related diseases, offering potential avenues for therapeutic interventions to mitigate the impact of chronic inflammation on overall health in the elderly.
Reduced vaccine efficacy: One of the concerning consequences of immunosenescence is its impact on vaccine responses [43][44][48,49]. As the aging immune system becomes less responsive and adaptable, vaccines may be less effective in providing adequate protection [45][46][50,51]. This phenomenon is particularly relevant for diseases such as influenza and COVID-19, where vaccines are crucial for preventing severe illness and complications [47][48][49][52,53,54]. Reduced vaccine efficacy in older individuals not only places them at higher risk for vaccine-preventable diseases, but may also hinder efforts to establish herd immunity, as the collective protection of the population depends on a sufficient number of individuals developing immunity through vaccination.
Increased susceptibility to infections: Immunosenescence also renders older individuals more vulnerable to a wide range of infections [50][55]. The diminished immune responses, especially in the adaptive immune system, make it harder for the body to fend off invading pathogens effectively [44][49]. This heightened susceptibility not only leads to a higher incidence of common infections—like respiratory illnesses—but also increases the risk of developing severe and potentially life-threatening infections. In the context of the COVID-19 pandemic, older adults have been disproportionately affected, experiencing higher rates of severe illness and mortality [51][52][53][54][55][56,57,58,59,60]. Additionally, infections, such as pneumonia and urinary tract infections, become more prevalent in older age, often resulting in longer hospital stays and slower recovery times [56][57][61,62]. Furthermore, the increased susceptibility to infections can have a cascading effect on overall health, potentially exacerbating age-related comorbidities and contributing to an increased burden of illness in the elderly population.
Impact on cancer risk: One consequence of immunosenescence is the diminished ability of the immune system to surveil and eliminate cancerous cells efficiently. This compromised immunosurveillance facilitates the initiation and progression of various cancers in elderly individuals [58][59][60][63,64,65]. Furthermore, chronic viral infections, such as CMV, which becomes more prevalent with age, have been linked to an elevated risk of certain cancers. CMV, in particular, may exacerbate immunosenescence and contribute to the development of malignancies. In addition to promoting tumor growth, the proinflammatory environment induced by immunosenescence can support the proliferation of cancer cells and facilitate their spread. This complex interplay between immunosenescence, chronic infections, and cancer underscores the importance of understanding the role of aging immune system in oncogenesis.
Autoimmunity: Paradoxically, while immunity against infections weakens, the risk of autoimmune diseases increases with age [61][62][66,67]. This dual impact highlights the complexity of immunosenescence. Autoimmunity refers to a condition in which the immune system mistakenly targets and attacks the body’s healthy cells and tissues [63][68]. As individuals age, changes in immune regulation mechanisms can lead to a breakdown of self-tolerance, resulting in autoimmune reactions [6][64][6,69]. The interplay between immunosenescence and autoimmunity has implications for the development of autoimmune disorders in older populations [6][42][65][66][6,47,70,71]. Diseases like rheumatoid arthritis, systemic lupus erythematosus, and autoimmune thyroid diseases are more prevalent in elderly individuals, and immunosenescence is believed to contribute to these conditions [67][72].
In conclusion, immunosenescence is a complex and multifaceted process involving alterations in the function and composition of the immune system that have far-reaching implications for overall health. Revealing these hallmarks is essential for understanding the role of immunosenescence in health and disease, as well as its complex relationship with chronic viral infections like cytomegalovirus.
3. Cytomegalovirus: The Silent Companion of Aging
Cytomegalovirus, a member of the Herpesviridae family, is a ubiquitous human pathogen with a remarkable prevalence worldwide. It infects between 40% and 95% of the global population, depending on geographic region and socio-economic factors [10][11][12][10,11,12]. CMV is typically acquired early in life, with primary infection often occurring during childhood or adolescence. Importantly, the majority of CMV infections in immunocompetent individuals remain asymptomatic, resulting in a latent and often lifelong viral presence.
3.1. Mechanisms of Latency and Reactivation
After primary CMV infection, the virus enters a latent phase where it persists in specific organ sites, mainly in hematopoietic progenitor cells and cells within the myeloid lineage. During latency, active genome replication and the production of viral progeny are not detectable, but residual transcriptional activity can be identified in several viral gene loci. This phenomenon is sometimes referred to as “sleepless latency.” CMV’s ability to establish latency and reactivate intermittently is a key feature of its persistence in the host [68][73].
The molecular mechanisms responsible for CMV establishing latency and its reactivation remain areas of active research. Multiple studies have made progress towards understanding how human CMV regulates latency, particularly in CD34+ progenitor cells in the bone marrow [69][74]. It was proposed that latency may be achieved through specific mechanisms of transcriptional silencing that vary depending on the cell type. On the other hand, reactivation can be induced through pathways activated by common triggers such as inflammation, infection, and injury, which are found in multiple cell types. Additionally, the differentiation of myeloid cells into dendritic cells can contribute to reactivation [69][74]. This highlights the complex relationship between CMV and the host immune response, where the virus exploits cell type-specific gene regulation mechanisms to establish latency and spread infection throughout the body. The influence of the inflammatory environment associated with aging, including the presence of inflammatory cytokines such as IL-6 and TNF-α, might play a role in triggering CMV reactivation in older individuals, occasionally leading to low-level viremia.
Moreover, immunosenescence itself may create an environment that might not only facilitate chronic infections like CMV, but also contribute to the reactivation of latent viruses [69][70][74,75]. The bidirectional relationship between immunosenescence and CMV involves complex mechanisms that warrant in-depth exploration. As mentioned before, immunosenescence is closely linked to inflammaging, and CMV infection may intensify this inflammatory environment by promoting the secretion of proinflammatory cytokines. In turn, inflammaging further accelerates immunosenescence, creating a positive feedback loop. This proinflammatory milieu is conducive to CMV reactivation, as the virus thrives in inflammatory conditions.
Immunosenescence is characterized by the progressive decline in T-cell function, including exhaustion, a state where T cells lose their ability to respond effectively to persistent viral infections. CMV, known for establishing lifelong latency, takes advantage of the compromised T-cell responses during immunosenescence. The persistence of CMV-specific T cells, particularly CD8+ T cells, contributes to the chronic immune activation seen in aging individuals [70][75].
Additionally, age-related changes in immune cell populations, such as the expansion of memory T cells, contribute to the permissive environment for CMV reactivation. The altered balance of immune cells—a hallmark of immunosenescence—provides favorable conditions for latent viruses like CMV to undergo reactivation, leading to episodic shedding and potential clinical manifestations.
The bidirectional interaction also involves epigenetic modifications. Immunosenescence-associated changes in DNA methylation and histone modifications may influence the regulation of CMV genes during latency [6]. Conversely, CMV-induced alterations in host cell epigenetics can impact the overall aging process and immune responsiveness.
Furthermore, immunosenescence affects antigen-presentation pathways crucial for recognizing and responding to viral infection [70][75]. CMV, with its ability to manipulate host cell machinery, may exploit these alterations in order to evade immune surveillance during latency. This interplay between immunosenescence-related changes and CMV strategies underscores the complexity of their bidirectional relationship.
Understanding the bidirectional dynamics between immunosenescence and CMV reactivation is essential for unraveling the complexities of aging-related immune dysfunction. Further research should explore the molecular and cellular intricacies of this relationship, searching for potential therapeutic interventions that target both immunosenescence and CMV in order to promote healthier aging and mitigate age-associated diseases.
It is necessary to emphasize that our understanding of virus–host interactions remains constrained not just by the complex interplay between CMV and the immune system, but also by the absence of an animal model that replicates human CMV physiology, as well as gaps in our knowledge regarding numerous proteins encoded by the CMV genome [71][76]. Understanding these aspects of CMV, from its complex relationship with the aging immune system to the mechanisms of latency and reactivation, is essential in order to grasp its potential impact on human health, especially in the context of aging and age-related diseases.
3.2. Sites of CMV Latency
After the resolution of the primary infection, CMV establishes latency in various organs and tissues throughout the human body [72][77]. The latent CMV genome, residing in various organs, serves as the molecular foundation for CMV reactivation across multiple organ systems [73][74][78,79]. These sites of latency are where the virus remains dormant, without causing active infection or manifest symptoms. The exact sites of CMV latency in humans can be difficult to pinpoint with accuracy. Researchers often turn to primary human tissue and cell culture models, along with the utilization of animal models to study CMV latency. Despite some limitations, these approaches remain a valuable tool for researchers, allowing us to better understand the virus’s behavior and interactions within different anatomical sites [75][80].
CMV primarily establishes latency in peripheral blood monocytes and hematopoietic progenitor cells [76][77][81,82]. These cells serve as a reservoir for the virus, allowing it to persist in a latent state within the host (Figure 2).
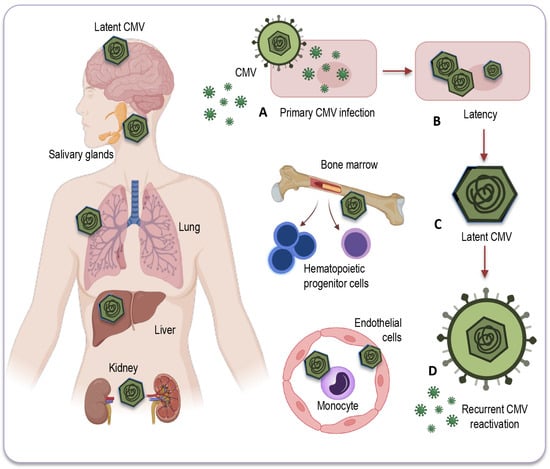
Figure 2. Potential sites of CMV latency. Following the resolution of the primary infection (A), CMV enters a latent state (B) within various human organs and tissues. This latent CMV genome (C) acts as the molecular basis for the reactivation of CMV (D) across multiple organ systems. CMV’s primary site of latency is within peripheral blood monocytes and hematopoietic progenitor cells, acting as a viral reservoir for prolonged persistence in the host. Additionally, CMV may be detected in a range of bodily tissues and organs, including the salivary glands, liver, kidneys, brain, and lungs, where reactivation and associated diseases may occur, particularly in individuals with compromised immune systems. CMV: Cytomegalovirus.
CMV can also be found in various tissues and organs throughout the body, including the liver [78][83], kidneys [69][74], spleen, and lungs [79][84], where it may reactivate and cause disease under certain conditions, especially in individuals with weakened immune systems. However, while CMV can infect some solid organs and tissues, they are not typically considered a site for CMV latency [73][78]. The exact nature of CMV’s presence and activity in solid organs remains a topic of ongoing research and discussion.
It is important, therefore, to note that some of the sites depicted in Figure 2, and briefly described below, can be hypothetical in humans, as identifying these locations with certainty can be challenging due to the complexities of CMV latency and analytical difficulties in precisely identifying them, relying on models and animal studies, the diagnostic tools and test methods available so far.
Myeloid progenitor cells: CD34+ progenitor cells in the bone marrow are considered important sites of CMV latency in humans. Reactivation from these cells can contribute to viral dissemination.
Monocytes and macrophages: Monocytes can serve as a reservoir for CMV, harboring the latent virus. CMV may reactivate in these cells and contribute to viral distribution.
Endothelial cells: CMV has also been found to establish latency in endothelial cells, which form the lining of blood vessels. This may have implications for vascular health and disease in elderly population.
Salivary glands: CMV can persist in the salivary glands, which contributes to its transmission through saliva. Reactivation in the salivary glands can lead to viral shedding and potential transmission of the infection.
Renal (kidney) tubules: CMV can establish latency in the renal tubules of the kidneys, which may play a role in the persistence of the virus in the body.
Liver: Some research has suggested that the liver could potentially serve as a site for CMV latency, but the details and prevalence of this phenomenon may vary among individuals. Further studies are needed to fully understand the extent and implications of CMV latency in the liver.
Neural tissue and brain: CMV’s ability to infect neural tissues, including the brain, has been less investigated. As CMV DNA has also been detected in non-immunocompromised individuals, the virus can undergo reactivation from latency, potentially leading to neurological and neurodegenerative disorders in elderly people.
It is important to note that CMV can possibly reactivate from these sites of latency when the host’s immune system is compromised or weakened. This reactivation can lead to the shedding of the virus and potentially cause symptomatic CMV infections, especially in individuals with weakened immune responses, such as the elderly or immunocompromised individuals. Exploring the sites of CMV latency and its reactivation conditions are vital for developing strategies to manage CMV infections and their potential impact on health, especially in the context of immunosenescence and age-related diseases. Understanding the sites of CMV latency and the mechanisms triggering its reactivation provides a crucial foundation for delving into the upcoming discussion on the intricate interplay between CMV and immunity.
4. The Complex Interplay: CMV and Immunity
4.1. Age-Related Modulation by CMV
The interaction of CMV with the human immune system is complex, multifaceted, and poorly understood [80][85]. While primary CMV infection in healthy individuals is usually subclinical or results in mild, flu-like symptoms, the immune response to CMV infection is robust and clear. It elicits a strong, long-lasting, and highly differentiated T-cell response, particularly in older children and adults [81][86]. CMV-specific memory T cells may become a significant portion of the immune repertoire, and this ongoing immune alteration may impact the overall functionality of the immune system.
In its role of shaping the immune system, CMV presents a unique and complex relationship with the aging immune system. While it is often considered a pathogen due to its potential to cause disease upon primary infection, particularly in infants and immunocompromised individuals, its role in healthy, immunocompetent adults is more nuanced [44][49]. This dual role is especially evident in CMV-positive young adults, where CMV infection can have both benefits and disadvantages. In particular, CMV infection in young adults typically results in highly differentiated and sensitized T cells specific to the virus, which can rapidly respond to CMV reactivation. This enhanced T-cell population could offer several advantages, including viral control, where CMV-specific T cells can efficiently control CMV reactivation, preventing symptomatic infections. Moreover, CMV-specific T cells may cross-react with other pathogens, providing an immunological benefit when encountering new infections. However, the presence of a highly differentiated T-cell population may also have drawbacks. The continuous activation and differentiation of CMV-specific T cells can lead to immune exhaustion and impaired immune response [82][83][87,88].
In the context of aging, the relationship between CMV and the immune system is even more complicated. On the one hand, CMV-specific T cells persist for decades in response to latent CMV infection, maintaining an elevated, specialized, and vigilant population of immune cells. The circulating CMV-specific T cells are often characterized by markers of senescence, such as the loss of CD28 expression, the accumulation of KLRG1 and CD57 markers, and telomere shortening. These hallmarks are typical of immunosenescence and can, in part, be attributed to CMV [84][89]. On the other hand, the chronic interaction of CMV with the aging immune system raises questions about potential negative consequences. Some evidence suggests that CMV-specific T cells may exhibit features of exhaustion and decline in functionality, which could further contribute to immunosenescence, ultimately affecting the host’s ability to respond effectively to other infections and possibly impacting overall health in aged individuals.
In various studies, it has been widely acknowledged that cytomegalovirus plays a significant role in driving immunosenescence. As mentioned before, one of the prominent features of immunosenescence is the expansion of CD28- T cells, which has been equally recognized as a hallmark of chronic CMV infection. CD28, a co-stimulatory molecule on naïve CD4 and CD8 T cells, is permanently lost during antigen-driven differentiation to a terminal phenotype, indicating replicative senescence [84][89]. While aging has historically been considered a key factor in this expansion, it is now clear that CMV infection may be a primary driver of this phenomenon. It has been shown that CMV-seropositive individuals exhibit up to a twelve-fold increase in CD4+CD28− T cells and a two-fold increase in CD8+CD28− T cells compared to CMV-seronegative individuals. Remarkably, this effect is only marginally influenced by age when CMV infection is present [84][85][89,90].
The CD8+ T-cell response to CMV is notably extensive, targeting a wide range of viral peptides. This response exhibits the unique characteristic of a continuous, long-term expansion of antigen-specific CD8 memory T cells, a phenomenon known as memory inflation. In fact, the CD8+ T-cell response to specific epitopes of this virus can constitute up to 20% of the total memory T-cell population, and the cumulative response to all CMV epitopes in humans is estimated to occupy 50% or more of the entire CD8+ memory T-cell pool [86][91]. This substantial and progressive response has been linked to the accumulation of dysfunctional CMV-specific T cells and potentially shorter lifespans in octogenarians, although the precise nature of this association remains unclear.
Intriguing insights into the complexity of the immune response to CMV in the elderly were demonstrated by Hadrup et al. [87][92]. They observed significant expansions of CD8+ T-cell clones specific to dominant CMV peptides in the majority of octogenarians and nonagenarians in a Swedish population. Notably, these expansions tended to become more pronounced with advancing age. Surprisingly, among individuals who lived to very old age, a different trend emerged. Their T-cell responses to CMV were comparatively lower, and some specific T-cell clonotypes responsive to the virus were even lost [87][92]. These findings suggest the interesting possibility that survival into extreme old age, including centenarians and beyond, may be linked to an immune system that is less preoccupied with persistent infections.
The concept of extreme longevity and its potential correlation with a diminished emphasis of the immune system on persistent infections opens a captivating avenue for exploration. The observation that individuals reaching very old age exhibit lower T-cell responses to CMV, coupled with the loss of specific responsive T-cell clonotypes [87][92], suggests a unique immune landscape in this demographic. This divergence from the typical pattern seen in aging individuals prompts a critical inquiry: could the reduction in immune responsiveness to persistent infections be a distinctive feature of those who achieve extreme longevity? Exploring this notion may unravel novel insights into the complex dynamics between aging, immune function, and the ability to reach remarkable lifespans. Investigating whether an immune system less preoccupied with persistent infections contributes to the remarkable resilience observed in individuals who defy the conventional boundaries of aging holds promise for opening new insights into the complex dynamics between aging, immune function, and the ability to achieve extraordinary lifespans.
Thus, CMV’s role in shaping immunity, especially in the context of aging, remains multifaceted. On one hand, it can drive immunosenescence, contributing to age-related pathologies, while, on the other, it may enhance immune responses against certain infections, mostly in young individuals. This dual role emphasizes the need for a comprehensive understanding of the interplay between CMV and immunity. The understanding of the immune system’s dynamics in extreme old age may also open new avenues for exploring the interplay between CMV, longevity, and immune function.
4.2. Interplay between CMV and Immunosenescence: Modulatory Effect of Genetics and Lifestyle
In general, immunosenescence exhibits considerable heterogeneity among individuals, and understanding these diverse trajectories is crucial. This requires a comprehensive exploration, recognizing the influence of various factors, including genetics and lifestyle, in modulating the impact of CMV on immunosenescence [23][88][28,93]. Factors such as genetic variations among individuals can influence their susceptibility to infections and the efficiency of their immune responses. Some individuals may carry genetic factors that make them more resilient or more vulnerable to CMV. Genetic predispositions may determine aspects of the immune system’s function, such as the effectiveness of specific immune cells in recognizing and combating the virus.
Genetic diversity contributes to variability in immune system function [88][89][90][93,94,95]. Different individuals may have variations in genes related to immune response pathways, cytokine production, and other immune-related functions. These genetic differences can impact how the body responds to CMV infection [89][91][94,96]. For example, genes involved in antigen presentation, such as those encoding human leukocyte antigens (HLA), may determine how effectively the immune system presents viral antigens to T cells. Genetic diversity in these genes can affect the recognition of CMV by the immune system. The genes encoding immune receptors, such as those on T cells, may play a crucial role in recognizing and responding to CMV. Genetic variations in these receptor genes can affect the specificity and strength of the immune response. It is known that interferons are key players in antiviral defense. Genetic variations can impact the efficiency of interferon responses to CMV, affecting the ability to control viral replication. Furthermore, individuals’ variations in genes related to inflammatory pathways may influence the degree of inflammation triggered by CMV. Excessive inflammation can further contribute to immunosenescence and age-related diseases.
Additionally, lifestyle factors, including diet, exercise, and overall health practices, further contribute to this complexity [92][93][97,98]. They can modulate the effects of CMV on the immune system and overall health. Epigenetic modifications, which can be influenced by lifestyle factors, play a crucial role in regulating gene expression. Lifestyle choices such as diet, stress, and environmental exposures can modify epigenetic marks, influencing how genes related to immune function respond to CMV and other infections. Lifestyle factors such as unhealthy diet, lack of physical activity, and exposure to environmental toxins can contribute to chronic inflammation [93][98]. Genetic predispositions to inflammatory responses may further exacerbate the impact of CMV and contribute to the development of age-related diseases.
Thus, unraveling the interplay between CMV, genetics, and lifestyle is essential for a more nuanced comprehension of immunosenescence trajectories, shedding light on personalized approaches to mitigate its effects and promote healthy aging. The interplay between genetics and lifestyle further underscores the complexity of the host–virus interaction, and highlights the importance of adopting a holistic approach to health. From another perspective, this understanding may contribute to the development of tailored approaches for addressing the impact of CMV on the aging immune system.
The following will provide a concise introduction to the intricate mechanisms by which CMV may actively contribute to the exacerbation of inflammation and accelerate the aging process.
5. Role of CMV in Fueling Inflammation and Aging
Beyond its impact on immunosenescence, CMV has increasingly gained attention for its complex role in the shaping of proinflammatory conditions within the host [94][99], contributing to the development of inflammaging, a state characterized by chronic low-grade inflammation that accompanies aging [8][41][8,46]. What mechanisms may facilitate CMV in inducing such a proinflammatory environment?
CMV infection has been linked to the activation of specific signaling pathways associated with inflammation [94][95][99,100]. Notably, CMV can stimulate the nuclear factor-kappa B (NF-κB) pathway, a key regulator of proinflammatory gene expression. Activation of NF-κB leads to the production of inflammatory mediators [86][91] and can activate innate immune responses by triggering pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs). This recognition leads to the production of proinflammatory cytokines, like IL-6 and TNF-α, which are known key players in inflammation. CMV infection can alter immune cell profiles and functions [96][101]. For instance, as repeatedly discussed before, CMV is associated with an expansion of CD28− T cells, which are functionally less effective but produce more proinflammatory cytokines, fueling inflammation.
Furthermore, CMV can induce senescent cells to produce SASP (senescence-associated secretory phenotype) factors, which include proinflammatory cytokines and chemokines. These factors contribute to a chronic state of inflammation that characterizes inflammaging. As discussed earlier, CMV can also exacerbate immunosenescence, contributing to chronic inflammation. This weakened immune function, coupled with the presence of CMV, may lead to persistent inflammation [97][98][102,103]. Additionally, CMV-specific T cells, while still highly cytotoxic, can produce proinflammatory cytokines. This can further contribute to persistent inflammation, especially in older individuals [99][104].
Taken together, CMV-induced immune alterations can similarly lead to a proinflammatory milieu, with increased production of inflammatory cytokines like IL-1β, IL-6, IL-8, and TNF-α. These cytokines are known drivers of inflammation and can lead to the activation of various immune and non-immune cells, further perpetuating a proinflammatory state. This can potentially initiate a detrimental cycle, resulting in further T-cell exhaustion and dysfunction, thus exacerbating the immune system’s limitations in effectively countering other infections and boosting the progression of immunosenescence. These conditions may collectively create a proinflammatory milieu in CMV-infected individuals, which has implications for age-related diseases, including cardiovascular diseases and cancer, and may even play a role in responses to acute infections like SARS-CoV-2. Therefore, the relationship between CMV and inflammation is a multifaceted and dynamic one, with implications for both immune function and age-related pathologies. Understanding these mechanisms is crucial for unraveling the complex interplay between CMV and inflammation in the context of aging and age-associated conditions.
