Pituitary adenomas is a type of brain tumor with diverse behaviors and complexities. About half of pituitary adenomas are known to secrete specific hormones, most frequently prolactin, growth hormone, or adrenocorticotropic hormone. Despite being histologically benign, these tumors can cause significant endocrine disturbances, leading to considerable morbidity and potentially shortening lifespan. Due to their pathophysiological endocrine secretion and proximity to critical neural and vascular structures, hormone-secreting pituitary adenomas require comprehensive management.
- pituitary adenomas
- molecular mechanisms
- targeted therapies
- immunotherapy
- biomarkers
- epigenetics
1. Introduction
23. Epigenetics of Pituitary Adenomas
2.1. Introduction to the Role of Epigenetics in Tumor Development—Specific Changes Associated with Pituitary Adenomas
3.1. Introduction to the Role of Epigenetics in Tumor Development—Specific Changes Associated with Pituitary Adenomas
Epigenetic mechanisms are crucial in mammalian development and maintaining tissue-specific gene expression. Disruptions in these processes can lead to changes in gene functionality and malignant cellular transformation. Global epigenetic alterations are now recognized as a significant hallmark of cancer. Cancer, traditionally viewed as a genetic disorder, is increasingly understood to involve both genetic mutations and epigenetic anomalies [5][17]. Epigenetic phenomena such as nucleosome remodeling through histone modifications, DNA methylation, and microRNA (miRNA)-mediated gene targeting play critical roles in regulating biochemical pathways vital to tumorigenesis. Moreover, mutations in genes that regulate epigenetics have strengthened the connection between epigenetics and cancer [6][18]. Histone modifications in pituitary tumors have been linked to increased p53 expression and longer progression-free survival. Sirtuins, a class of proteins, have a higher expression in growth hormone-expressing adenomas than in nonfunctional ones and show an inverse correlation with tumor size in somatotrophs. The elevation of citrullinating enzymes has been proposed as an early marker in the pathogenesis of prolactinomas [7][21]. Epigenetic therapies targeting DNA methyltransferases (DNMTs) and histone deacetylases (HDACs) have been suggested to reactivate the expression of epigenetically silenced genes, potentially enhancing tumor cell sensitivity to conventional treatments like chemotherapy and radiotherapy [8][22]. miRNAs, small non-coding single-stranded RNAs that regulate gene expression post-transcriptionally, have been increasingly implicated in pituitary tumorigenesis. Variations in miRNA expression are linked with the development of pituitary tumors. Depending on the context, these miRNAs can act as oncosuppressors or oncogenes, underscoring their pivotal role in the onset and progression of these tumors [9][10][11][23,24,25].2.2. Potential Therapeutic Avenues Targeting Epigenetic Modifications
3.2. Potential Therapeutic Avenues Targeting Epigenetic Modifications
The role of the histone deacetylase (HDAC) family, especially HDAC2 and HDAC3 from Class I, in the development of pituitary tumors is not fully understood. Studies have shown that HDAC2 and 3 are more highly expressed in clinically non-functioning pituitary adenomas compared to normal pituitary tissues, as evidenced by RT-PCR and immunohistochemical staining (IHC) analyses. In a different research stream, LaPierre et al. explored the role of miR-7a2 in lactotrophic cell development and prolactin hormone synthesis, following preliminary data suggesting a connection between miR-7a2 deficiency and reduced prolactin expression. Contrary to initial expectations, they found that miR-7a2 knockout led to an early increase in lactotroph cell proliferation and prolactin production during embryonic development, but this was followed by a decrease in hormone production in adulthood. In contrast, the overexpression of miR-7a2 was linked to delayed lactotroph development. Further experiments in various mouse pituitary cell types and rat prolactinoma cells showed that miR-7a2 exerts its effects by inhibiting its target gene Raf1, a known promoter of prolactin production. This finding underscores the complex regulation of prolactin production. However, more research is necessary to fully elucidate miR-7a2’s role in hyperprolactinemia and evaluate its potential as a therapeutic target [9][23].34. Transcriptomic Insights into Pituitary Adenomas
3.1. Overview of Transcriptomics and Its Significance in Cancer Biology
4.1. Overview of Transcriptomics and Its Significance in Cancer Biology
Transcriptome profiling has emerged as a crucial tool in oncology over recent decades, providing significant prognostic and predictive insights for cancer management. This approach has transformed cancer perception from a primarily histopathological and organ-centric perspective to a more molecularly based classification, enabling more personalized diagnostics and treatment strategies. The development of single-cell transcriptomic sequencing represents a major leap in this area, offering a detailed analysis of tumor ecosystems and deepening the understanding of tumorigenesis at the cellular level [12][13][14][27,28,29]. Single-cell RNA sequencing (scRNA-seq) has been particularly influential in cancer cell research, uncovering new facets of cancer biology, including the cancer stem-cell concept, treatment resistance mechanisms, and the dynamics of cancer metastasis [14][15][29,30]. Analyses of altered transcriptional profiles in pituitary adenomas have revealed significant differences beyond genes involved in somatic copy number alterations (SCNAs). Tumors with disrupted genomes show more variability in gene expression compared to quieter tumors. This suggests that the processes leading to unstable genomes also result in diverse transcriptomes [16][33]. In prolactin-producing pituitary adenomas (PRL-PAs), research has identified widespread genomic copy number amplifications, which correlate with transcriptomic changes in this tumor subtype. High copy number variations (CNVs) in PRL-PA are associated with increased prolactin production, drug resistance, and proliferation, potentially through key genes like BCAT1. This research provides insights into the effects of genomic CNVs on transcriptomes and clinical outcomes in PRL-PA, highlighting potential therapeutic targets [17][34]. Pituitary adenomas have been categorized into three molecular clusters based on the transcription factor guiding their terminal differentiation. The first cluster, driven by NR5A1, includes clinically non-functioning pituitary adenomas, encompassing gonadotrophinomas and null cell adenomas. The second cluster, influenced by TBX19, contains clinically evident ACTH adenomas and silent corticotroph adenomas. The third cluster, driven by POU1F1, includes TSH-, PRL-, and GH-adenomas [18][35].3.2. Implications for Targeted Therapy Based on Transcriptomic Data
4.2. Implications for Targeted Therapy Based on Transcriptomic Data
Recent research has highlighted the importance of understanding the multiomic profiles of pituitary neuroendocrine tumors (PitNETs) to develop molecularly targeted therapies, especially for those resistant to current treatments. This aligns with the broader trend of applying precision medicine in cancer treatment [19][37]. In corticotrophinomas, a significant finding was the discovery of a somatic mutational hotspot in the ubiquitin-specific peptidase 8 (USP8) gene in nearly half of these tumors. This gene produces a protein that impedes the downregulation of the epidermal growth factor receptor (EGFR), leading to its prolonged activation. EGFR, crucial for corticotroph function, is highly expressed in Cushing’s pituitary tumors and stimulates ACTH synthesis. The mutation in corticotrophinomas increases USP8 activity, preventing EGFR degradation and maintaining its stimulatory effect [20][21][38,39]. In acromegaly treatment, the drug pasireotide has demonstrated greater efficacy than first-generation somatostatin analogs like octreotide or lanreotide, suggesting that it could become a new standard for patients who do not respond well to first-generation analogs [22][40].45. Immunological Aspects of Pituitary Adenomas
4.1. Introduction to the Immune Response in Intracranial Tumors
5.1. Introduction to the Immune Response in Intracranial Tumors
An investigation into the immunological landscape of pituitary adenomas, using gene expression data, revealed a dominance of specific immune cell types. The most prevalent infiltrating immune cells in these tumors are M2 macrophages, followed by resting CD4+ memory T cells and mast cells. Silent pituitary tumors particularly show a higher fraction of M2 macrophages compared to other subtypes. Conversely, Cushing’s pituitary tumors, including overt and subclinical cases, have a higher proportion of CD8+ T cells than growth hormone (GH) tumors, prolactinomas, hyperthyroid tumors, and silent tumors [23][43]. The tumor microenvironment immune composition (TMIC) in pituitary adenomas consists of myeloid cells like tumor-associated macrophages, dendritic cells, and lymphocytes including T cells and B cells (Figure 1). The interplay between these infiltrating immune cells, their secretions, the tumor, and its host is intricate, and it can lead to either tumor-promoting or anti-tumor effects [24][44].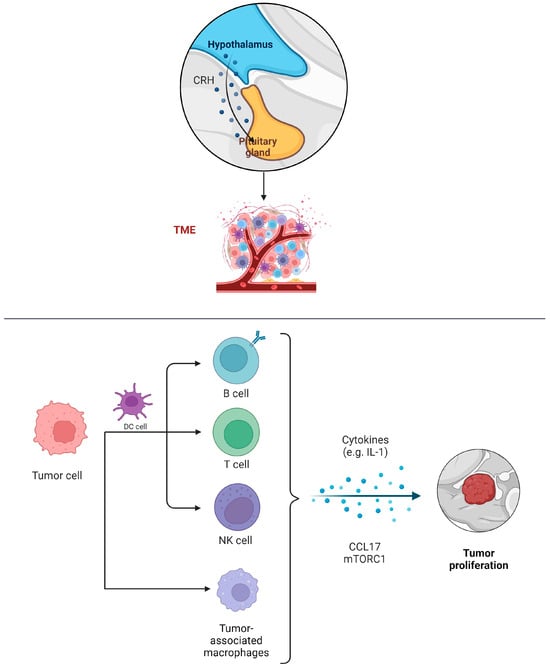
4.2. Opportunities and Challenges for Immunotherapy in Pituitary Adenomas
5.2. Opportunities and Challenges for Immunotherapy in Pituitary Adenomas
56. The Tumor Microenvironment in Pituitary Adenomas
5.1. Exploring the Microenvironment’s Contribution to Pituitary Adenoma Progression
6.1. Exploring the Microenvironment’s Contribution to Pituitary Adenoma Progression
In the tumor microenvironment, the presence and activity of non-tumoral cells significantly affect tumor proliferation, invasiveness, and angiogenesis. Fibroblasts, usually in a dormant mesenchymal state, can become activated in response to various stimuli, contributing to both normal physiological processes like wound healing and pathological processes such as tumor progression. Tumor-associated fibroblasts (TAFs) are abnormally activated due to growth factors and cytokines produced by cancer cells [27][52]. These activated TAFs secrete a variety of molecules that are key in remodeling the extracellular matrix and facilitating tumor growth, invasiveness, metastasis, and resistance to treatment [28][29][53,54]. Interleukin-6 (IL-6) plays a role in hormone release, tumor growth and proliferation, and the production of angiogenic factors, particularly vascular endothelial growth factor-A (VEGF-A) [30][55]. It has been found that IL-6 stimulates the growth of GH3 rat pituitary tumor cells while inhibiting the growth of normal rat pituitary cells. IL-6 can reach the pituitary through systemic circulation and is also produced within the pituitary, having paracrine effects.5.2. Strategies to Target and Modulate the Tumor Microenvironment for Therapeutic Advantage
6.2. Strategies to Target and Modulate the Tumor Microenvironment for Therapeutic Advantage
The enhanced understanding of the immune tumor microenvironment (TME) has been pivotal in the development and clinical implementation of immuno-checkpoint inhibitors (ICIs), significantly transforming the treatment of various cancers in the past decade [31][59]. In light of these developments, ICIs are being explored as a new therapeutic option for aggressive pituitary adenomas/PitNETs and in the rarer instances of pituitary carcinomas [24][44]. Tumor-associated fibroblasts (TAFs) within the TME of pituitary adenomas play a crucial biological role. Cytokines released from TAFs can impact both tumoral and non-tumoral cells, including macrophages. This interaction may enhance tumor invasiveness and influence angiogenesis and epithelial-to-mesenchymal pathways in PAs. IL-6 and CCL2 have been identified as key factors in this process. Intriguingly, the somatostatin analogue pasireotide has been found to inhibit the secretions of TAFs, suggesting a potential anti-tumoral effect of somatostatin analogues (SSAs) by directly targeting TAFs and thus altering the TME in PAs [32][60] (Figure 2).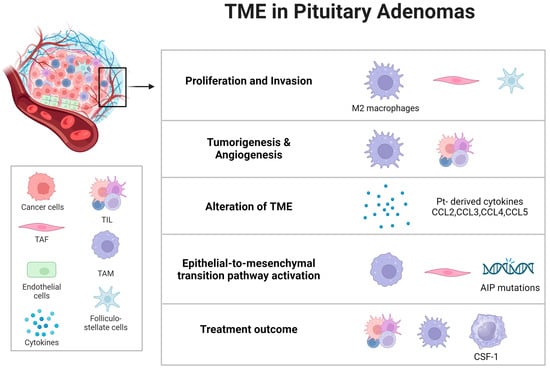
67. Biomarkers: The Future of Diagnosis and Treatment
6.1. The Importance of Biomarkers in Oncology
7.1. The Importance of Biomarkers in Oncology
6.2. Exploring Potential Biomarkers Specific to Intracranial Tumors and Pituitary Adenomas
7.2. Exploring Potential Biomarkers Specific to Intracranial Tumors and Pituitary Adenomas
Several biomarkers have been identified that possess predictive value for managing pituitary tumors, particularly concerning their clinical and radiological characteristics. The cell cycle is regulated by key components such as cyclins, cyclin-dependent kinases (CDKs), and their inhibitors (CDKIs). There are two main families of CDKIs: the INK family (including INK4a/p16, INK4b/p15, INK4c/p18, and INK4d/p19) and the WAF/KIP family (comprising WAF1/p21, KIP1/p27, and KIP2/p57). The progression through the cell cycle is largely controlled by fluctuations in the levels of cyclins and CDKIs, which are regulated via programmed degradation by the ubiquitin–proteasome system [36][67]. In adrenocorticotrophic hormone-secreting pituitary tumors, the roles of matrix metalloproteinase-9 (MMP-9), pituitary tumor-transforming gene (PTTG), and high mobility group A 2 (HMGA2) in tumor development are well established. However, their relationships with tumor recurrence following transsphenoidal adenomectomy remain unclear. Vascular endothelial growth factor plays a vital role in angiogenesis, a process critical in both developmental stages and pathological conditions in pituitary tumors. A significant amount of preclinical and clinical research has highlighted the importance of anti-VEGF therapy in treating pituitary tumors [37][38][51,69].6.3. Role of Biomarkers in Early Diagnosis and Targeted Therapies
7.3. Role of Biomarkers in Early Diagnosis and Targeted Therapies
Pituitary adenomas exhibit varied behaviors, and predicting their potential for aggressive or malignant transformation is complex. Biomarkers are increasingly recognized as vital in this context, including a range of factors such as chromosomal changes, microRNAs, markers of cellular proliferation, oncogenes, tumor suppressor genes, growth factors and their receptors, and elements related to angiogenesis or cell adhesion [39][71]. Recent research has identified three RNA subtypes, messenger-RNA, long non-coding RNA, and micro-RNA, that show correlations with PitNETs. However, their exploration in liquid biopsies remains limited, with only a few studies conducted so far.78. Old and Emerging Targets for Therapy
7.1. Historical Treatment Options: A Static Landscape
8.1. Historical Treatment Options: A Static Landscape
78.1.1. Analysis of Traditional Treatments over the Past 20 Years
In the past two decades, there has been significant progress in the treatment approaches for intracranial tumors. Radiation therapy, traditionally key in treating malignant and aggressive intracranial tumors, has proven its effectiveness by extending patient survival rates and improving tumor control. Radiotherapy (RT) is a core element in the management of malignant tumors and is increasingly being applied to benign conditions. The effectiveness of low to intermediate doses of RT has been extensively studied. However, the application of post-operative radiotherapy (PORT) continues to be debated, in spite of numerous trials and meta-analyses conducted over many years [40][75]. The execution of large-scale, multicenter therapeutic trials remains a challenge. Future research is expected to increasingly depend on data from translational clinical trials, particularly those using canine intracranial tumor models. Continuous data collection and analysis regarding the natural biology and clinical progression of specific tumor types and grades are crucial for evaluating therapeutic methods. This requires a minimum histologic diagnosis for publication.78.1.2. The Exceptions: Pituitary Adenomas and the Alternative Treatments
Pituitary adenomas constitute around 15% of all brain tumors, and their detection is increasingly common, largely due to the widespread use of magnetic resonance imaging. Surgical intervention is the primary treatment for most of these tumors. The effectiveness of dopaminergic agonists and somatostatin receptor ligands (SRLs) in treating specific types of pituitary adenomas, notably prolactinomas and growth hormone excess, is well established. Over the past decade, there has been an increase in the application of new multi-receptor binding SRLs for acromegaly and Cushing’s disease treatment [41][77]. Functional pituitary adenomas, which can cause morbidity through hormone hypersecretion, are often effectively managed with medical therapies aimed at inhibiting pituitary hormone secretion or the response of target organs. These medical interventions are non-invasive and mitigate the anatomical and potentially permanent risks associated with surgical and radiation treatments. However, aside from prolactinomas, typically medical therapies are not curative and are unlikely to fully eliminate the adenoma. The main objective of these therapies is to attain biochemical control, and treatment risks vary based on the specific medication. Considering that these therapies might be prolonged or indefinite, understanding patient tolerability is essential [4][14]. Radiotherapy has been proven effective in managing pituitary adenomas, achieving 97.9% radiological control and 93.6% biochemical control over a median six-year follow-up period post radiotherapy [42][78]. Techniques like intensity-modulated radiation therapy, stereotactic radiosurgery, and proton beam radiation therapy, which focus energy beams precisely on the tumor to minimize damage to adjacent healthy tissues, are employed [43][79].7.2. Targeted Therapies
8.2. Targeted Therapies
Pituitary adenomas are linked with several related disorders, including prolactinoma, acromegaly, Cushing’s disease, and non-functioning pituitary adenoma. Treatment often requires a combination of surgery, medical therapies (such as dopamine agonists or somatostatin receptor ligands), and radiotherapy, due to their significant impact on patient mortality, morbidity, and quality of life [44][45][82,83]. Transsphenoidal surgery is usually the first-line treatment for pituitary tumors, except for prolactinomas. However, the tumor’s local invasiveness may complicate surgical resection, occasionally necessitating initial medical therapy [46][84].
Prolactinomas, the most common type of secretory pituitary tumors, can cause symptoms from prolactin oversecretion, localized mass effects, or both [47][85]. Traditionally, dopamine agonists have been the primary treatment, with cabergoline effectively normalizing prolactin levels in most patients and inducing tumor shrinkage in many. The surgical resection of microprolactinomas and encapsulated macroprolactinomas can achieve remission rates similar to cabergoline treatment [48][86]. Acromegaly, a chronic disease caused mainly by growth hormone-secreting PitNETs, can lead to severe health issues and increased mortality if not properly managed. Transsphenoidal surgery is preferred for GH-secreting PitNETs, with medical therapy, especially long-acting somatostatin analogs, used when surgery is not possible or incomplete, achieving control in about half of the patients [49][87]. Cushing’s disease, caused by an ACTH-secreting pituitary tumor, is the most common form of Cushing’s syndrome and requires prompt diagnosis and treatment to improve outcomes [50][88]. Surgery is the initial therapy, but when it is not curative, medical therapy becomes a significant secondary option. New drugs and formulations are being investigated for their efficacy and safety in treating Cushing’s disease [51][89]. Non-functioning pituitary adenomas, benign tumors that do not cause hormonal hypersecretion, are primarily treated with surgery, but recurrence rates are high [52][91]. Post surgery, dopamine agonists have been explored to prevent recurrence, showing some initial promise but diminishing effectiveness over time. The role of medical therapy in preventing NFPA regrowth remains limited [53][92]. In managing pituitary adenomas, including prolactinoma, acromegaly, Cushing’s disease, and non-functioning pituitary adenoma, transsphenoidal surgery is typically the primary treatment, except for prolactinomas. Sometimes, the tumor’s invasiveness may necessitate initial medical intervention. Surgical resection maintains its status as a core strategy in managing brain tumors (BM), with its effectiveness in BM surgery being well established. A critical aspect of surgical intervention is the method employed, wherein en bloc resection, the removal of the tumor in a single piece, has been shown to offer better local control compared to piecemeal tumor resection. This approach also involves the minimal removal of adjacent normal brain tissue [54][93]. En bloc resection is notable for its potential to decrease intraoperative tumor spread relative to piecemeal resection. Patel et al. observed that en bloc resection does not correlate with an increase in complications or adverse functional outcomes [55][94]. Most pituitary adenomas can be effectively managed with current medical treatments, surgical interventions, and, in some cases, radiotherapy. However, gonadotroph adenomas are particularly challenging due to the lack of effective medical treatments. For prolactinomas, the use of dopamine agonists has been a primary treatment strategy since the 1970s. The initial use of bromocriptine later shifted to cabergoline, which has been effective in regulating prolactin levels in up to 85% of patients and reducing tumor size in about 80% of cases. The surgical removal of both microprolactinomas and larger macroprolactinomas, when performed by neurosurgeons specialized in pituitary disorders, has achieved remission rates comparable to those obtained with cabergoline therapy. For particularly aggressive prolactinomas and metastasized PitNETs, a comprehensive treatment approach is recommended. This includes higher doses of cabergoline, surgical intervention, radiation therapy (preferably stereotactic radiosurgery when possible), and temozolomide administration. While DAs remain effective for most prolactinoma cases, the success rates of transsphenoidal surgical methods have significantly improved recently, especially with the expertise of specialized surgeons [56][98].