Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Elena Puerta.
Sirtuin 2 (SIRT2), one of the seven members of the sirtuin family, has emerged as a potential regulator of aging and age-related pathologies since several studies have demonstrated that it shows age-related changes in humans and different animal models. A detailed analysis of the relevant works addressing this topic shows that the changes that occur in SIRT2 with aging seem to be opposite in the brain and in the periphery.
- aging
- brain
- epigenetics
- inflammation
- neurodegenerative diseases
- sirtuin 2
1. SIRT2 and Aging
In the last decades, with the increase of life expectancy, there has been a demographic growth in the elderly population, a tendency that is expected to continue. According to the World Health Organization, it is expected that from 2015 to 2050 the world’s population over 60 years old will almost double, raising from 12% to 22% [1]. As the tendency to disease increases with age, the focus on healthy aging and its research is becoming more relevant. Considering the aging process is very diverse, if we aim to understand it, the influence of genetics should not be ignored. Gene expression can be altered in many levels such as DNA replication, transcription, RNA translation, and post-translational modifications of proteins. In this context, epigenetics could also be a piece of the puzzle. Epigenetics is defined as “the study of changes in gene function that are mitotically and/or meiotically heritable and that do not entail a change in DNA sequence” [2]. These changes occur through several mechanisms, all of which can be modulated by environmental, physiological, and pathological processes. Some of the predominant epigenetic mechanisms are ATP-dependent chromatin-remodeling complexes, non-coding RNAs, covalent modifications of DNA bases and histone modifications [3].
In particular, histone modifications have been widely studied. Histones are octameric proteins around which DNA strands wrap, conforming the chromatin. These DNA-binding proteins provide a structural support to DNA, that can be extended or compacted depending on the modifications (methylation, acetylation, phosphorylation, and ubiquitination) that take place in their tails. Notably, histone acetylation on the lysine residues reduces chromatin condensation becoming more accessible to transcription factors, and activating the transcription of genes located in the affected region. This results in an increase gene expression. Histone acetyltransferases (HAT) and histone deacetylases (HDAC) are crucial in these epigenetic modifications since they are responsible for dynamic histone acetylation and deacetylation, respectively, tuning the level of transcripts (for a review, see [4]) (Figure 1).
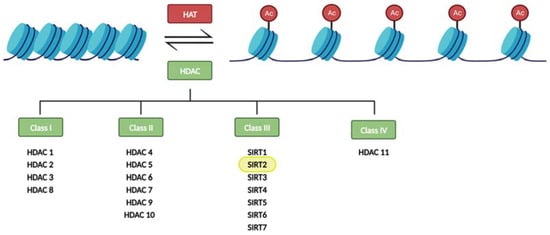
Figure 1. Regulation of histone acetylation levels by histone acetyl transferases (HATs) and histone deacetylases (HDACs). Sirtuins constitute the class III of HDACs.
Eighteen HDAC proteins are classified into four classes named I–IV. Class III HDACs, also known as sirtuins, are nicotinamide adenine nucleotide- (NAD+) dependent, while the other three classes require zinc as a cofactor. Sirtuins also differ from other HDACs in their unique ability to catalyze ADP-ribosylation [5,6][5][6].
Sirtuins are present in every living species and widely distributed along the tissues [7]. Besides deacetylating histone proteins, they are also key in the control of many physiological processes in mammals. For instance, they take part in the regulation of the cell cycle, antioxidant protection, inflammation, neurogenesis, and various metabolic pathways. Thus, their functions are often related to stress response, aging, and general homeostasis [8,9][8][9].
To date, seven isoforms of sirtuins (SIRT1-7) have been described in mammals. They all differ in their functions among other reasons due to diverse terminal regions, subcellular location, enzymatic activities, and substrates [10,11][10][11]. Regarding their subcellular location, SIRT1 is mostly located in the nucleus, with the ability to migrate to the cytosol. SIRT2, on the other hand, is the only sirtuin that is predominantly cytosolic, although it has the capacity to shuttle to the nucleus and mitochondria. SIRT3, SIRT4, and SIRT5 are mitochondrial sirtuins, being SIRT3 and SIRT5 able to migrate to the cytoplasm. SIRT6 is found in the nucleus, associated with chromatin, and SIRT7 in the nucleolus and nucleus [9,12,13,14][9][12][13][14].
Although their functions are very diverse and many studies are still needed to understand their role in each process, it has been described that SIRT1 is involved in inflammation, oxidative stress, cell proliferation, and apoptosis [15,16][15][16], while the other nuclear sirtuins, SIRT6 and SIRT7, participate in DNA repair [17] and regulation of gene transcription [18], respectively. Mitochondrial sirtuins SIRT3, SIRT4, and SIRT5 are involved in the response to oxidative stress and metabolic pathways inside this organelle [19]. SIRT2 has been involved in multiple functions regulating gene expression and many metabolic pathways [20].
Since the interest in sirtuins is growing, research is being carried out on all the members of the sirtuin family in different experimental models, tissues, and conditions, leading to new findings that intend to clarify their physiological and pathological relevance. Specifically, several recent studies have described age-related changes in SIRT2 in different organs and tissues [21,22,23,24][21][22][23][24]. This has led to the hypothesis that SIRT2 could play a key role in the aging process. However, these changes are not the same in all tissues, since an increase [25,26,27][25][26][27] or a decrease [28,29,30][28][29][30] in its expression has been described depending on the tissue analyzed. Therefore, a detailed understanding of the function of SIRT2 with aging in each cell type is necessary to determine if it is an interesting target for the treatment of diseases associated with aging.
2. CNS SIRT2 Expression in Aging
SIRT2 is the most abundant member of the sirtuin family in the CNS, notably in the cortex, striatum, hippocampus, and spinal cord [12]. In recent years, this fact has exponentially increased interest in SIRT2 in neuroscience research in order to decipher its implication in aging and age-related neurodegenerative disorders. The first study on this topic was published by Maxwell and colleagues in 2011 [25]. In this study, the authors found that three isoforms predicted for SIRT2 (SIRT2.1, SIRT2.2, and SIRT2.3) are expressed in the brain. They analyzed young adult (4–5-month-old) and aged (19–22-month-old) C57BL/6 mice and found an age-related accumulation of the isoform SIRT2.3 in spinal cord extracts and cortices. As a result, total SIRT2 levels had a modest but significant increase in aged mice. Notably, they detected an association between the local accumulation of SIRT2 protein and areas of reduced tubulin acetylation in cell bodies and neurites which could be affecting neuronal function. More recently, the behavioral and molecular consequences of the overexpression of SIRT2.3 in the hippocampus have been addressed [45][31]. According to this work, the overexpression of SIRT2.3 does not result in relevant behavioral or molecular changes in control mice. However, in a mouse model of accelerated aging, the SAMP8 model, SIRT2.3 overexpression worsened learning and memory performance and increased the expression of the pro-inflammatory cytokine IL-1β. Based on these results, the increase of SIRT2.3 in aged brains does not seem to induce or prevent senescence, but it could play a part in the progression of age-related processes together with other risk factors. In line with Maxwell’s findings, a study checked for SIRT2 level variations in various brain areas of female Wistar rats at 3, 12, and 24 months old. An increase in Sirt2 mRNA and protein levels was observed, but exclusively in the occipital lobe. This increase was paired with a significant enhancement in deacetylated FOXO3a (Forkhead Box, class O3a) transcription factor, a substrate deacetylated by SIRT2, in the same region [24]. Supporting this data, a recent study, analyzing the cortex and hippocampus of 3- and 22-month-old Wistar albino male rats, has shown an increase in SIRT2 and FOXO3a brain levels during the aging process, accompanied by an increase in oxidative stress and apoptosis [27]. In addition, authors randomly administered melatonin, which is reported to have antioxidant, anti-apoptotic, and anti-aging properties, and is physiologically reduced in aging; the SIRT2 inhibitor salermide, or DMSO as a control to both young and aged rats. They described a reduction in SIRT2 and FOXO3 protein levels in the hippocampus but not in the cortex of aged rats treated with melatonin. Interestingly, salermide administration to aged rats led to the inhibition of SIRT2 and FOXO3 in both regions. Considering these results and the functions attributed to melatonin in aging, the authors suggest that SIRT2 and FOXO3 could play a key role in oxidative stress and apoptosis [27]. Indeed, the FOXO transcription factors are regulated by post-translational modifications, and SIRT2-mediated deacetylation of FOXO3a promotes its ubiquitination and degradation [46][32]. In fact, SIRT2 deacetylates FOXO3a and stimulates its translocation to the nucleus, therefore inducing apoptosis [47][33]. In addition, exposure to oxidative stress upregulates FOXO3a in the hippocampus [48][34], enhanced levels of SIRT2 lead to cell death, and the inhibition of SIRT2 results in a reduction of oxidative stress and apoptosis [22,49,50,51,52][22][35][36][37][38]. In agreement with these observations, an increase in FOXO3a activity has been found in neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease [53,54][39][40]. However, the reason why the increase in SIRT2 is only observed in certain brain regions and the physiological consequences of these changes need to be further investigated. Supporting the notion that elevated SIRT2 levels in the CNS are deleterious, another study has shown an upregulation of SIRT2 in the brain of a D-galactose-induced aging rat model [55][41]. In fact, as a result of D-galactose administration, the expression of the pro-inflammatory cytokines interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) increased, while the autophagic marker Beclin-1 was downregulated. Noteworthy, metformin supplementation induced an anti-aging effect, downregulating the expressions of SIRT2, IL-6, and TNF-α, whereas increasing Beclin-1 expression. The authors state that metformin promotes the activation of autophagy and reduces inflammation, hence restoring the antioxidant status and improving brain aging [55][41]. In line with these results, the implication of SIRT2 in autophagy has also been described in different studies. It has been shown that upregulated SIRT2 interferes with autophagy efficiency and promotes protein accumulation under proteasome inhibition, intensifying proteinopathy-related cytotoxicity [56][42]. Consistently, lowered SIRT2 increases autophagy levels [57][43]. Taking into account that, in the context of neurodegenerative disorders, inadequate autophagy induces neuronal cell death while activated autophagy is neuroprotective, these results further justify the deleterious consequences of age-related increases in SIRT2 in the CNS. In a different publication, Diaz-Perdigon and colleagues [26] compared SIRT2 protein and mRNA levels in 2- and 9-month-old male SAMR1 and SAMP8 mice. In both models, they found a significant increase in hippocampal SIRT2 protein in aged animals, with no significant differences between both strains. Therefore, they pointed out SIRT2 as a possible biomarker of the aging process. However, this increase did not correlate with changes in Sirt2 mRNA, which according to the authors, indicates protein accumulation rather than an increase in its synthesis. In contrast, there were no significant changes in the protein expression in the frontal cortex and striatum. Interestingly, in order to understand the physiological consequences of the observed SIRT2 increase, they administered the SIRT2 inhibitor 33i to 5- and 8-month-old SAMP8 and SAMR1 mice. Authors conclude that early SIRT2 inhibition improves age-related cognitive decline and prevents neuroinflammation in SAMP8 mice. However, the inhibition of SIRT2 once the aging phenotype is well established (in 8-month-old SAMP8 mice) cannot reverse age-induced behavioral and molecular changes [26]. These results point to SIRT2 inhibition as a promising therapeutic target to prevent age-related cognitive decline. In agreement with all these studies, it has been recently published that SIRT2 protein expression increases gradually with aging in the cortex and hippocampus isolated from 3-, 6-, 12-, and 24-month-old C57BL/6 wild type (WT) mice [58][44]. Interestingly, the authors show that, at the same time, SIRT1 expression decreases gradually; thus, the SIRT2:SIRT1 ratio gradually increases with age. In an attempt to understand how the changes in SIRT1 and SIRT2 levels may affect the vulnerability of the neurons to a neurotoxic insult, SH-SY5Y neuroblastoma cells were transfected with empty vector, flag-tagged SIRT1 or SIRT2, and then treated with Aβ42 oligomers. They found that Aβ substantially increased cell death when transfecting cells with an empty vector, whereas SIRT1 overexpression largely restored the cell damage by Aβ. On the other hand, SIRT2 overexpression reduced the survival of Aβ42-treated cells compared to untreated cells [58][44]. Together, these data support the notion that SIRT1 and SIRT2 have inverse effects on neuron viability; SIRT1 protects against neurotoxicity, while SIRT2 promotes it. The findings of the studies mentioned above point out an increase of SIRT2 in the CNS during the aging process. However, another study performed by Kireev and colleagues showed differing results when testing male Wistar rats [59][45]. In this case, the researchers found a significant age-related decrease of Sirt2 mRNA accompanied by an increase in gene and protein levels of pro-apoptotic markers (Bax and Bad) in the dentate gyrus comparing 2- and 22-month-old animals [59][45]. Noteworthy, in this case, growth hormone treatment reduced the pro/anti-apoptotic ratio to levels observed in young animals and also increased SIRT2 levels, which was accompanied by a reduction in apoptosis and enhanced survival markers in this part of the hippocampus. In general terms, most of the studies collected in this section agree in concluding that SIRT2 seems to be increased in the CNS with aging (Table 1) and that this increase seems to be harmful by promoting oxidative stress and neurodegeneration. Therefore, based on these conclusions, SIRT2 inhibition or different strategies aimed at counteracting age-related increases in SIRT2 could be considered good therapeutic options for age-related diseases.Table 1.
Sirtuin 2 expression variations in the aging CNS.
| Authors and Year | Analyzed Model | Sample | Sirtuin 2 Expression with Aging | |
|---|---|---|---|---|
| Specie | Ages Compared in Months | |||
| Maxwell et al., 2011 [25] | ||||
Table 2.
Sirtuin 2 expression changes with aging in the periphery.
| Authors and Year | Analyzed Model | Sample | Sirtuin 2 Expression with Aging | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Specie | Ages Compared | ||||||||
| C57BL/6 mouse | 4–5 vs. 19–22 | Spinal cord and cortex | Increase | ||||||
| Kireev et al., 2013 [59 | |||||||||
| Chambers et al., 2007 [21] | C57BL/6 mouse | 2- vs. 21-month-old | HSCs isolated from BM | Decrease | |||||
| ][45] | |||||||||
| Yudoh et al., 2015 [60][46] | Male Wistar rat | 2 vs. 22 | Hippocampus | Human |
22- to 66-year-old(dentate gyrus) | Decrease | |||
| PBMCs | Decrease | Braidy et al., 2015 [24] | Female Wistar rat | 3 vs. 12 vs. 24 | Occipital lobe | ||||
| Luo et al., 2019 [29] | C57BL/6 mouse | Increase | |||||||
| 3- vs. 24-month-old | HSCs isolated from BM | Decrease | Garg et al., 2017 [55][41] | Male Wistar rat | 4 vs. 24 | Whole brain | Increase | ||
| Wongchitrat et al., 2019 [28] | Human | 25- to 35-year-old vs. ≥65-year-old | Peripheral blood (plasma) | Increase | Diaz-Perdigon et al., 2020 [26] | Male SAMR1 and SAMP8 mice | 2 vs. 9 | Hippocampus | Increase |
| Lehallier et al., 2019 [23] | Human | 18- to 95-year-old | Peripheral blood (plasma) | Decrease | Keskin-Atkan et al., 2022 [27] | Male Wistar rat | |||
| He et al., 2020 [ | 3 vs. 22 | 44][ | Hippocampus and cortex | Increase | |||||
| 47] | Male C57BL/6 mouse | 3- vs. 24-month-old | Macrophages isolated from BM | Decrease | Li et al., 2023 [58][44] | C57BL/6 mouse | 3 vs. 6 vs. 12 vs. 24 | Hippocampus and cortex | Increase |
3. Peripheral SIRT2 Expression in Aging
Considering that the expression of SIRT2 is very extensive in the periphery, several works have also addressed the changes that occur in its expression in different peripheral cell types (Table 2| Ye et al., 2023 | ||||
| [ | ||||
| 30 | ||||
| ] | ||||
| Cynomolgus macaque | 4- to 6- vs. 18- to 21-year-old | Cardiomyocytes | Decrease | |
| Zhang et al., 2023 [61][48] | C57BL/6 mouse | 4- vs. 24-month-old | Aorta and VSMCs | Decrease |
BM: bone marrow; HSC: hematopoietic stem cell; PBMC: peripheral blood mononuclear cell; VSMC: vascular smooth muscle cell.
In 2007, Chambers and colleagues [21] carried out a study using highly purified bone marrow hematopoietic stem cells (HSC) from 2- and 21-month-old C57BL/6 mice. They analyzed the expression of more than 14,000 genes identifying 1500 that were age-induced and 1600 that were age-repressed. As expected, the up-regulated genes were associated with the stress response, inflammation, and protein aggregation, whereas the down-regulated group was marked by genes involved in maintaining genomic integrity and chromatin remodeling. Among them, SIRT2 was found to be significantly reduced with aging [21]. In their conclusions, authors highlight the epigenetic perspective of aging, which elucidates the diversity of the effects of age at the molecular, cellular, and organ levels. In agreement with this study, a few years later, Luo et al. [29] also studied HSCs isolated from bone marrow of 3-month-old and 24-month-old C57BL/6 mice. Again, they found a reduction in Sirt2 mRNA levels in old mice compared to the young ones [29]. Aiming to elucidate whether these changes are cause or consequence of aging and to assess the functions of SIRT2 in HSCs, they analyzed HSCs in WT versus SIRT2 knockout (KO) mice. They observed that old SIRT2 KO mice had fewer HSCs in bone marrow compared to old WT mice. In addition, they found a decrease in lymphoid and an increase in myeloid cells in the peripheral blood of aged SIRT2 KO mice, which implies that SIRT2 has an age-dependent effect on HSC maintenance and hematopoiesis. They also demonstrate that SIRT2 promotes HSC survival upon the activation of the NLRP3 inflammasome, and suggest, for the first time, that SIRT2 could modify the NLRP3 activity at the post-transcriptional level. Thus, reduced SIRT2 expression in aged HSCs explains the age-induced upregulation of the NLRP3 inflammasome. Supporting this hypothesis, NLRP3 downregulation or SIRT2 overexpression counteracted the functional decline of HSC with aging [29].
More recently, He and colleagues [44][47] measured Sirt2 mRNA levels in macrophages isolated from bone marrow of male C57BL/6 mice, and they found that the expression in this cell type was reduced in 24-month-old compared to 3-month-old animals. In agreement with the aforementioned observations, they demonstrate that SIRT2 deacetylates NLRP3 leading to the inactivation of the NLRP3 inflammasome. This serves as evidence supporting the physiological significance of the acetylation switch in the NLRP3 inflammasome, thereby regulating inflammation associated with aging and influencing glucose homeostasis. In addition, they demonstrate that 2-year-old SIRT2 KO mice fed a chow diet exhibit metabolic alterations, insulin resistance, and peripheral chronic inflammation. These results indicate that insulin sensitivity maintenance and repression of NLRP3 inflammasome activation during aging necessitate SIRT2 [44][47]. This research reveals a mechanism of inflammaging and points out that aging-associated conditions can be reversed by upregulating SIRT2 or promoting NLRP3 deacetylation.
Supporting these studies, another publication has also reported an inverse correlation between SIRT2 levels and age in peripheral blood mononuclear cells (PBMC) of healthy humans [60][46]. The authors point out the involvement of SIRT2 in aging biology and suggest that it may be a potential biomarker for monitoring health conditions and aging [60][46]. However, in another study, the analysis of SIRT2 expression in the peripheral blood of healthy adults (25–35 years old) and elderly people (65 years old and over) led to the opposite conclusion: a significant age-related increase in SIRT2 mRNA levels was found [28].
Due to the high prevalence of cardiovascular diseases in aging, several recent studies have also focused on studying whether SIRT2 could be playing an important role in the correct functioning of this system. Ye et al. [30] have compared hearts from aged (18- to 21-year-old) and young (4- to 6-year-old) cynomolgus macaques. They found that the size of the aged monkey’s cardiomyocytes doubled the size of the younger ones. They also revealed an increased cardiac fibrosis and staining of senescence-associated β-galactosidase with age. In order to determine the underlying molecular mechanisms of these phenotypic differences, they carried out mass spectrometry-based proteomics in cardiomyocytes. As expected, they found upregulation of proteins involved in pro-inflammatory response, blood clotting, and fibrosis; and downregulation of proteins in pathways related to protein synthesis, mitochondrial function, and lipid metabolism, in aged versus young hearts. Among them, they observed, for the first time, a sex-independent and age-related decrease of the SIRT2 protein in heart samples. Interestingly, the cardioprotective role of SIRT2 was supported by in vivo experiments in mouse models. Intramyocardial injection of lentiviruses expressing SIRT2 resulted in an improved cardiac dysfunction in aging [30]. This study supports the notion that SIRT2 is a key protein in the periphery; specifically, it exhibits cardioprotective effects and can be proposed as a potential therapeutic target against age-related cardiomyocyte hypertrophy and associated cardiac dysfunction.
In agreement with these conclusions, another recent study has also proposed that SIRT2 may serve as a potential therapeutic target for vascular rejuvenation [61][48]. The study describes that among the sirtuin family, SIRT2 is the most abundant in human and mouse aortas, an expression which is reduced with aging. Interestingly, old SIRT2 KO mice show accelerated vascular aging (arterial stiffness and constriction–relaxation dysfunction, accompanied by aortic remodeling, collagen deposition, and inflammation), which correlates with mitochondrial oxidative stress and transcriptome reprogramming. Moreover, they also show that SIRT2 is also relevant for aging and related vascular diseases in humans. Using a public proteome dataset researchers found that plasmatic SIRT2 is decreased with aging and is a valuable predictor of age-related aortic diseases in humans [23].
Supporting the beneficial consequences of maintaining peripheral SIRT2 expression in different organs in aging, it has been demonstrated that SIRT2 could have a key role in the mechanisms underlying caloric restriction. Caloric restriction, without malnutrition, is the most effective and reproducible physiological intervention promoting longevity from yeast to mammals (reviewed in [62][49]). It is the most consistent non-genetic and non-pharmacological approach to extend lifespan, acting through a reduction in insulin and insulin-like growth factor, and an increase of insulin sensitivity [55][41]. In particular, SIRT2 expression increased in the kidney and white adipose tissue of mice in response to caloric restriction [47][33]. It is hypothesized that some of the beneficial consequences of caloric restriction could be mediated by this increase in SIRT2 since it deacetylates BubR1, a promoter of a healthy lifespan that is physiologically reduced in aging. It has been described that mice overexpressing BubR1 live longer than the hypomorphic ones, which in addition show signs of accelerated aging. Noteworthy, by keeping lysine-668 of BubR1 deacetylated, SIRT2 promotes BubR1 stability therefore increasing the lifespan of BubR1 deficient mice [63][50].
Together, these data coincide in demonstrating that SIRT2 decreases in the periphery with age and highlight the importance of maintaining its expression to delay aging and age-related diseases and further investigating the molecular mechanisms underlying this beneficial effect.
References
- Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 13 October 2023).
- Wu, C.T.; Morris, J.R. Genes, Genetics, and Epigenetics: A Correspondence. Science 2001, 293, 1103–1105.
- Pagiatakis, C.; Musolino, E.; Gornati, R.; Bernardini, G.; Papait, R. Epigenetics of Aging and Disease: A Brief Overview. Aging Clin. Exp. Res. 2021, 33, 737–745.
- Hamilton, J.P. Epigenetics: Principles and Practice. Dig. Dis. 2011, 29, 130–135.
- Park, S.-Y.; Kim, J.-S. A Short Guide to Histone Deacetylases Including Recent Progress on Class II Enzymes. Exp. Mol. Med. 2020, 52, 204–212.
- Bahl, S.; Seto, E. Regulation of Histone Deacetylase Activities and Functions by Phosphorylation and Its Physiological Relevance. Cell. Mol. Life Sci. 2021, 78, 427–445.
- Shoba, B.; Lwin, Z.M.; Ling, L.S.; Bay, B.-H.; Yip, G.W.; Kumar, S.D. Function of Sirtuins in Biological Tissues. Anat. Rec. 2009, 292, 536–543.
- Sharma, A.; Mahur, P.; Muthukumaran, J.; Singh, A.K.; Jain, M. Shedding Light on Structure, Function and Regulation of Human Sirtuins: A Comprehensive Review. 3 Biotech. 2023, 13, 29.
- Ziętara, P.; Dziewięcka, M.; Augustyniak, M. Why Is Longevity Still a Scientific Mystery? Sirtuins—Past, Present and Future. Int. J. Mol. Sci. 2023, 24, 728.
- Chen, X.; Lu, W.; Wu, D. Sirtuin 2 (SIRT2): Confusing Roles in the Pathophysiology of Neurological Disorders. Front. Neurosci. 2021, 15, 614107.
- Carafa, V.; Rotili, D.; Forgione, M.; Cuomo, F.; Serretiello, E.; Hailu, G.S.; Jarho, E.; Lahtela-Kakkonen, M.; Mai, A.; Altucci, L. Sirtuin Functions and Modulation: From Chemistry to the Clinic. Clin. Epigenetics 2016, 8, 61.
- Wang, Y.; Yang, J.; Hong, T.; Chen, X.; Cui, L. SIRT2: Controversy and Multiple Roles in Disease and Physiology. Ageing Res. Rev. 2019, 55, 100961.
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F.; et al. The Sirtuin Family in Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 402.
- Liu, G.; Park, S.-H.; Imbesi, M.; Nathan, W.J.; Zou, X.; Zhu, Y.; Jiang, H.; Parisiadou, L.; Gius, D. Loss of NAD-Dependent Protein Deacetylase Sirtuin-2 Alters Mitochondrial Protein Acetylation and Dysregulates Mitophagy. Antioxid. Redox Signal. 2017, 26, 849–863.
- Shen, P.; Deng, X.; Chen, Z.; Ba, X.; Qin, K.; Huang, Y.; Huang, Y.; Li, T.; Yan, J.; Tu, S. SIRT1: A Potential Therapeutic Target in Autoimmune Diseases. Front. Immunol. 2021, 12, 779177.
- Manjula, R.; Anuja, K.; Alcain, F.J. SIRT1 and SIRT2 Activity Control in Neurodegenerative Diseases. Front. Pharmacol. 2021, 11.
- Yang, Y.; Zhu, M.; Liang, J.; Wang, H.; Sun, D.; Li, H.; Chen, L. SIRT6 Mediates Multidimensional Modulation to Maintain Organism Homeostasis. J. Cell Physiol. 2022, 237, 3205–3221.
- Tong, Z.; Wang, Y.; Zhang, X.; Kim, D.D.; Sadhukhan, S.; Hao, Q.; Lin, H. SIRT7 Is Activated by DNA and Deacetylates Histone H3 in the Chromatin Context. ACS Chem. Biol. 2016, 11, 742–747.
- Ji, Z.; Liu, G.-H.; Qu, J. Mitochondrial Sirtuins, Metabolism, and Aging. J. Genet. Genom. 2022, 49, 287–298.
- Zhu, C.; Dong, X.; Wang, X.; Zheng, Y.; Qiu, J.; Peng, Y.; Xu, J.; Chai, Z.; Liu, C. Multiple Roles of SIRT2 in Regulating Physiological and Pathological Signal Transduction. Genet. Res. 2022, 2022, 9282484.
- Chambers, S.M.; Shaw, C.A.; Gatza, C.; Fisk, C.J.; Donehower, L.A.; Goodell, M.A. Aging Hematopoietic Stem Cells Decline in Function and Exhibit Epigenetic Dysregulation. PLoS Biol. 2007, 5, e201.
- Anwar, T.; Khosla, S.; Ramakrishna, G. Increased Expression of SIRT2 Is a Novel Marker of Cellular Senescence and Is Dependent on Wild Type P53 Status. Cell Cycle 2016, 15, 1883–1897.
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yousef, H.; Moran Losada, P.; Berdnik, D.; Keller, A.; Verghese, J.; et al. Undulating Changes in Human Plasma Proteome Profiles across the Lifespan. Nat. Med. 2019, 25, 1843–1850.
- Braidy, N.; Poljak, A.; Grant, R.; Jayasena, T.; Mansour, H.; Chan-Ling, T.; Smythe, G.; Sachdev, P.; Guillemin, G.J. Differential Expression of Sirtuins in the Aging Rat Brain. Front. Cell Neurosci. 2015, 9, 167.
- Maxwell, M.M.; Tomkinson, E.M.; Nobles, J.; Wizeman, J.W.; Amore, A.M.; Quinti, L.; Chopra, V.; Hersch, S.M.; Kazantsev, A.G. The Sirtuin 2 Microtubule Deacetylase Is an Abundant Neuronal Protein That Accumulates in the Aging CNS. Hum. Mol. Genet. 2011, 20, 3986–3996.
- Diaz-Perdigon, T.; Belloch, F.B.; Ricobaraza, A.; Elboray, E.E.; Suzuki, T.; Tordera, R.M.; Puerta, E. Early Sirtuin 2 Inhibition Prevents Age-Related Cognitive Decline in a Senescence-Accelerated Mouse Model. Neuropsychopharmacology 2020, 45, 347–357.
- Keskin-Aktan, A.; Akbulut, K.G.; Abdi, S.; Akbulut, H. SIRT2 and FOXO3a Expressions in the Cerebral Cortex and Hippocampus of Young and Aged Male Rats: Antioxidant and Anti-Apoptotic Effects of Melatonin. Biol. Futur. 2022, 73, 71–85.
- Wongchitrat, P.; Pakpian, N.; Kitidee, K.; Phopin, K.; Dharmasaroja, P.A.; Govitrapong, P. Alterations in the Expression of Amyloid Precursor Protein Cleaving Enzymes MRNA in Alzheimer Peripheral Blood. Curr. Alzheimer Res. 2019, 16, 29–38.
- Luo, H.; Mu, W.-C.; Karki, R.; Chiang, H.-H.; Mohrin, M.; Shin, J.J.; Ohkubo, R.; Ito, K.; Kanneganti, T.-D.; Chen, D. Mitochondrial Stress-Initiated Aberrant Activation of the NLRP3 Inflammasome Regulates the Functional Deterioration of Hematopoietic Stem Cell Aging. Cell Rep. 2019, 26, 945–954.e4.
- Ye, Y.; Yang, K.; Liu, H.; Yu, Y.; Song, M.; Huang, D.; Lei, J.; Zhang, Y.; Liu, Z.; Chu, Q.; et al. SIRT2 Counteracts Primate Cardiac Aging via Deacetylation of STAT3 That Silences CDKN2B. Nat. Aging 2023, 3, 1269–1287.
- Sola-Sevilla, N.; Ricobaraza, A.; Hernandez-Alcoceba, R.; Aymerich, M.S.; Tordera, R.M.; Puerta, E. Understanding the Potential Role of Sirtuin 2 on Aging: Consequences of SIRT2.3 Overexpression in Senescence. Int. J. Mol. Sci. 2021, 22, 3107.
- Wang, F.; Chan, C.-H.; Chen, K.; Guan, X.; Lin, H.-K.; Tong, Q. Deacetylation of FOXO3 by SIRT1 or SIRT2 Leads to Skp2-Mediated FOXO3 Ubiquitination and Degradation. Oncogene 2012, 31, 1546–1557.
- Wang, F.; Nguyen, M.; Qin, F.X.-F.; Tong, Q. SIRT2 Deacetylates FOXO3a in Response to Oxidative Stress and Caloric Restriction. Aging Cell 2007, 6, 505–514.
- Gómez-Crisóstomo, N.P.; Rodríguez Martínez, E.; Rivas-Arancibia, S. Oxidative Stress Activates the Transcription Factors FoxO 1a and FoxO 3a in the Hippocampus of Rats Exposed to Low Doses of Ozone. Oxid. Med. Cell Longev. 2014, 2014, 805764.
- Lynn, E.G.; McLeod, C.J.; Gordon, J.P.; Bao, J.; Sack, M.N. SIRT2 Is a Negative Regulator of Anoxia–Reoxygenation Tolerance via Regulation of 14-3-3 ζ and BAD in H9c2 Cells. FEBS Lett. 2008, 582, 2857–2862.
- Nie, H.; Hong, Y.; Lu, X.; Zhang, J.; Chen, H.; Li, Y.; Ma, Y.; Ying, W. SIRT2 Mediates Oxidative Stress-Induced Apoptosis of Differentiated PC12 Cells. Neuroreport 2014, 25, 838–842.
- Sarikhani, M.; Mishra, S.; Desingu, P.A.; Kotyada, C.; Wolfgeher, D.; Gupta, M.P.; Singh, M.; Sundaresan, N.R. SIRT2 Regulates Oxidative Stress-Induced Cell Death through Deacetylation of c-Jun NH2-Terminal Kinase. Cell Death Differ. 2018, 25, 1638–1656.
- She, D.T.; Wong, L.J.; Baik, S.-H.; Arumugam, T.V. SIRT2 Inhibition Confers Neuroprotection by Downregulation of FOXO3a and MAPK Signaling Pathways in Ischemic Stroke. Mol. Neurobiol. 2018, 55, 9188–9203.
- Pino, E.; Amamoto, R.; Zheng, L.; Cacquevel, M.; Sarria, J.-C.; Knott, G.W.; Schneider, B.L. FOXO3 Determines the Accumulation of α-Synuclein and Controls the Fate of Dopaminergic Neurons in the Substantia Nigra. Hum. Mol. Genet. 2014, 23, 1435–1452.
- Qin, W.; Zhao, W.; Ho, L.; Wang, J.; Walsh, K.; Gandy, S.; Pasinetti, G.M. Regulation of Forkhead Transcription Factor FoxO3a Contributes to Calorie Restriction-Induced Prevention of Alzheimer’s Disease-Type Amyloid Neuropathology and Spatial Memory Deterioration. Ann. N.Y. Acad. Sci. 2008, 1147, 335–347.
- Garg, G.; Singh, S.; Singh, A.K.; Rizvi, S.I. Antiaging Effect of Metformin on Brain in Naturally Aged and Accelerated Senescence Model of Rat. Rejuvenation Res. 2017, 20, 173–182.
- Gal, J.; Bang, Y.; Choi, H.J. SIRT2 Interferes with Autophagy-Mediated Degradation of Protein Aggregates in Neuronal Cells under Proteasome Inhibition. Neurochem. Int. 2012, 61, 992–1000.
- Inoue, T.; Nakayama, Y.; Li, Y.; Matsumori, H.; Takahashi, H.; Kojima, H.; Wanibuchi, H.; Katoh, M.; Oshimura, M. SIRT2 Knockdown Increases Basal Autophagy and Prevents Postslippage Death by Abnormally Prolonging the Mitotic Arrest That Is Induced by Microtubule Inhibitors. FEBS J. 2014, 281, 2623–2637.
- Li, N.; Bai, N.; Zhao, X.; Cheng, R.; Wu, X.; Jiang, B.; Li, X.; Xue, M.; Xu, H.; Guo, Q.; et al. Cooperative Effects of SIRT1 and SIRT2 on APP Acetylation. Aging Cell 2023, 22, e13967.
- Kireev, R.A.; Vara, E.; Tresguerres, J.A.F. Growth Hormone and Melatonin Prevent Age-Related Alteration in Apoptosis Processes in the Dentate Gyrus of Male Rats. Biogerontology 2013, 14, 431–442.
- Yudoh, K.; Karasawa, R.; Ishikawa, J. Age-Related Decrease of Sirtuin 2 Protein in Human Peripheral Blood Mononuclear Cells. Curr. Aging Sci. 2015, 8, 256–258.
- He, M.; Chiang, H.-H.; Luo, H.; Zheng, Z.; Qiao, Q.; Wang, L.; Tan, M.; Ohkubo, R.; Mu, W.-C.; Zhao, S.; et al. An Acetylation Switch of the NLRP3 Inflammasome Regulates Aging-Associated Chronic Inflammation and Insulin Resistance. Cell Metab. 2020, 31, 580–591.e5.
- Zhang, Y.; Wang, X.; Li, X.-K.; Lv, S.-J.; Wang, H.-P.; Liu, Y.; Zhou, J.; Gong, H.; Chen, X.-F.; Ren, S.-C.; et al. Sirtuin 2 Deficiency Aggravates Ageing-Induced Vascular Remodelling in Humans and Mice. Eur. Heart J. 2023, 44, 2746–2759.
- Fontana, L.; Partridge, L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell 2015, 161, 106–118.
- North, B.J.; Rosenberg, M.A.; Jeganathan, K.B.; Hafner, A.V.; Michan, S.; Dai, J.; Baker, D.J.; Cen, Y.; Wu, L.E.; Sauve, A.A.; et al. SIRT2 Induces the Checkpoint Kinase BubR1 to Increase Lifespan. EMBO J. 2014, 33, 1438–1453.
More
