Parkinson’s disease is a neurodegenerative condition characterized by motor dysfunction resulting from the degeneration of dopamine-producing neurons in the midbrain. This dopamine deficiency gives rise to a spectrum of movement-related symptoms, including tremors, rigidity, and bradykinesia. While the precise etiology of Parkinson’s disease remains elusive, genetic mutations, protein aggregation, inflammatory processes, and oxidative stress are believed to contribute to its development. In this context, fatty acid-binding proteins (FABPs) in the central nervous system, FABP3, FABP5, and FABP7, impact α-synuclein aggregation, neurotoxicity, and neuroinflammation. These FABPs accumulate in mitochondria during neurodegeneration, disrupting their membrane potential and homeostasis. In particular, FABP3, abundant in nigrostriatal dopaminergic neurons, is responsible for α-synuclein propagation into neurons and intracellular accumulation, affecting the loss of mesencephalic tyrosine hydroxylase protein, a rate-limiting enzyme of dopamine biosynthesis.
- Parkinson’s disease
- tyrosine hydroxylase
- dopaminergic neurons
- fatty acid-binding protein
- α-synuclein
- mitochondria
- dementia with Lewy bodies
1. Physiological Function of FABP and Involvement in Neurodegenerative Diseases
| FABP Subfamily | Tissue Distribution | Expressed Cells | Ref |
|---|---|---|---|
| FABP1 (Liver FABP) |
Liver, intestine, kidney, pancreas | Hepatocytes, enterocytes | [33,34,35][31][32][33] |
| FABP2 (Intestinal FABP) |
Intestine | Enterocytes | [36,37,38][34][35][36] |
| FABP3 (Heart FABP) |
Heart, skeletal muscle, brain | Cardiomyocytes, myocytes, neurons | [18,39,40,41,42,43][13][37][38][39][40][41] |
| FABP4 (Adipocyte FABP) |
Adipose tissue, macrophages | Adipocytes, macrophages | [44,45,46,47][42][43][44][45] |
| FABP5 (Epidermal FABP) |
Epidermis, brain, adipose tissue | Keratinocytes, adipocytes, glial cells, neurons | [18,43,48,49,50][13][41][46][47][48] |
| FABP6 (Ileal FABP) |
Intestine | Enterocytes | [51,52,53,54][49][50][51][52] |
| FABP7 (Brain FABP) |
Brain, eye, kidney, mammary gland | Neural stem cells, oligodendrocytes, astrocytes, ependymal cells | [18,41,43,55,56][13][39][41][53][54] |
| FABP8 (Myelin FABP) |
Myelin-forming cells in the peripheral nervous system | Schwann cells, oligodendrocytes | [43,56][41][54] |
| FABP9 (Testis FABP) |
Testis | Salivary gland, mammary gland | [57,58][55][56] |
2. Pathology of Parkinson’s Disease and Current Issues
3. Therapeutic Potential of FABP-Targeting Drugs for Parkinson’s Disease
4. Diagnostic Potential of FABPs as a Prodromal Biomarker for Parkinson’s Diseases
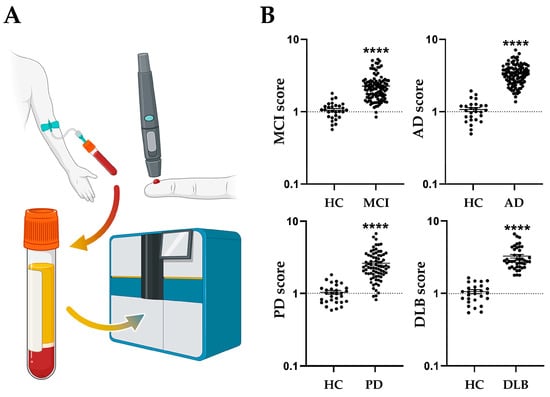
References
- Ockner, R.K.; Manning, J.A.; Poppenhausen, R.B.; Ho, W.K. A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science 1972, 177, 56–58.
- Veerkamp, J.H. Fatty acid transport and fatty acid-binding proteins. Proc. Nutr. Soc. 1995, 54, 23–37.
- Coe, N.R.; Bernlohr, D.A. Physiological properties and functions of intracellular fatty acid-binding proteins. Biochim. Biophys. Acta 1998, 1391, 287–306.
- Storch, J.; Thumser, A.E. The fatty acid transport function of fatty acid-binding proteins. Biochim. Biophys. Acta 2000, 1486, 28–44.
- Chmurzyńska, A. The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J. Appl. Genet. 2006, 47, 39–48.
- Smathers, R.L.; Petersen, D.R. The human fatty acid-binding protein family: Evolutionary divergences and functions. Hum. Genom. 2011, 5, 170–191.
- Zimmerman, A.W.; Veerkamp, J.H. New insights into the structure and function of fatty acid-binding proteins. Cell. Mol. Life Sci. 2002, 59, 1096–1116.
- Zimmerman, A.W.; van Moerkerk, H.T.; Veerkamp, J.H. Ligand specificity and conformational stability of human fatty acid-binding proteins. Int. J. Biochem. Cell Biol. 2001, 33, 865–876.
- Liu, R.Z.; Mita, R.; Beaulieu, M.; Gao, Z.; Godbout, R. Fatty acid binding proteins in brain development and disease. Int. J. Dev. Biol. 2010, 54, 1229–1239.
- Xu, B.; Chen, L.; Zhan, Y.; Marquez, K.N.S.; Zhuo, L.; Qi, S.; Zhu, J.; He, Y.; Chen, X.; Zhang, H.; et al. The Biological Functions and Regulatory Mechanisms of Fatty Acid Binding Protein 5 in Various Diseases. Front. Cell Dev. Biol. 2022, 10, 857919.
- Binas, B.; Danneberg, H.; McWhir, J.; Mullins, L.; Clark, A.J. Requirement for the heart-type fatty acid binding protein in cardiac fatty acid utilization. FASEB J. 1999, 13, 805–812.
- Schaap, F.G.; Binas, B.; Danneberg, H.; van der Vusse, G.J.; Glatz, J.F. Impaired long-chain fatty acid utilization by cardiac myocytes isolated from mice lacking the heart-type fatty acid binding protein gene. Circ. Res. 1999, 85, 329–337.
- Owada, Y.; Yoshimoto, T.; Kondo, H. Spatio-temporally differential expression of genes for three members of fatty acid binding proteins in developing and mature rat brains. J. Chem. Neuroanat. 1996, 12, 113–122.
- Owada, Y. Fatty acid binding protein: Localization and functional significance in the brain. Tohoku J. Exp. Med. 2008, 214, 213–220.
- Hanhoff, T.; Lucke, C.; Spener, F. Insights into binding of fatty acids by fatty acid binding proteins. Mol. Cell. Biochem. 2002, 239, 45–54.
- Armstrong, E.H.; Goswami, D.; Griffin, P.R.; Noy, N.; Ortlund, E.A. Structural basis for ligand regulation of the fatty acid-binding protein 5, peroxisome proliferator-activated receptor beta/delta (FABP5-PPARbeta/delta) signaling pathway. J. Biol. Chem. 2014, 289, 14941–14954.
- Mita, R.; Beaulieu, M.J.; Field, C.; Godbout, R. Brain fatty acid-binding protein and omega-3/omega-6 fatty acids: Mechanistic insight into malignant glioma cell migration. J. Biol. Chem. 2010, 285, 37005–37015.
- Perrin, R.J.; Woods, W.S.; Clayton, D.F.; George, J.M. Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J. Biol. Chem. 2001, 276, 41958–41962.
- Sharon, R.; Goldberg, M.S.; Bar-Josef, I.; Betensky, R.A.; Shen, J.; Selkoe, D.J. alpha-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 9110–9115.
- Sharon, R.; Bar-Joseph, I.; Frosch, M.P.; Walsh, D.M.; Hamilton, J.A.; Selkoe, D.J. The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson’s disease. Neuron 2003, 37, 583–595.
- Yakunin, E.; Loeb, V.; Kisos, H.; Biala, Y.; Yehuda, S.; Yaari, Y.; Selkoe, D.J.; Sharon, R. Alpha-synuclein neuropathology is controlled by nuclear hormone receptors and enhanced by docosahexaenoic acid in a mouse model for Parkinson’s disease. Brain Pathol. 2012, 22, 280–294.
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Fukushima, W.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Dietary fat intake and risk of Parkinson’s disease: A case-control study in Japan. J. Neurol. Sci. 2010, 288, 117–122.
- Pelsers, M.M.; Hanhoff, T.; Van der Voort, D.; Arts, B.; Peters, M.; Ponds, R.; Honig, A.; Rudzinski, W.; Spener, F.; de Kruijk, J.R.; et al. Brain- and heart-type fatty acid-binding proteins in the brain: Tissue distribution and clinical utility. Clin. Chem. 2004, 50, 1568–1575.
- Teunissen, C.E.; Veerhuis, R.; De Vente, J.; Verhey, F.R.; Vreeling, F.; van Boxtel, M.P.; Glatz, J.F.; Pelsers, M.A. Brain-specific fatty acid-binding protein is elevated in serum of patients with dementia-related diseases. Eur. J. Neurol. 2011, 18, 865–871.
- Basso, M.; Giraudo, S.; Corpillo, D.; Bergamasco, B.; Lopiano, L.; Fasano, M. Proteome analysis of human substantia nigra in Parkinson’s disease. Proteomics 2004, 4, 3943–3952.
- Oizumi, H.; Yamasaki, K.; Suzuki, H.; Hasegawa, T.; Sugimura, Y.; Baba, T.; Fukunaga, K.; Takeda, A. Fatty Acid-Binding Protein 3 Expression in the Brain and Skin in Human Synucleinopathies. Front. Aging Neurosci. 2021, 13, 648982.
- Wada-Isoe, K.; Imamura, K.; Kitamaya, M.; Kowa, H.; Nakashima, K. Serum heart-fatty acid binding protein levels in patients with Lewy body disease. J. Neurol. Sci. 2008, 266, 20–24.
- Kawahata, I.; Sekimori, T.; Oizumi, H.; Takeda, A.; Fukunaga, K. Using Fatty Acid-Binding Proteins as Potential Biomarkers to Discriminate between Parkinson’s Disease and Dementia with Lewy Bodies: Exploration of a Novel Technique. Int. J. Mol. Sci. 2023, 24, 13267.
- Mollenhauer, B.; Steinacker, P.; Bahn, E.; Bibl, M.; Brechlin, P.; Schlossmacher, M.G.; Locascio, J.J.; Wiltfang, J.; Kretzschmar, H.A.; Poser, S.; et al. Serum heart-type fatty acid-binding protein and cerebrospinal fluid tau: Marker candidates for dementia with Lewy bodies. Neurodegener. Dis. 2007, 4, 366–375.
- Backstrom, D.C.; Eriksson Domellof, M.; Linder, J.; Olsson, B.; Ohrfelt, A.; Trupp, M.; Zetterberg, H.; Blennow, K.; Forsgren, L. Cerebrospinal Fluid Patterns and the Risk of Future Dementia in Early, Incident Parkinson Disease. JAMA Neurol. 2015, 72, 1175–1182.
- Ockner, R.K.; Manning, J.A.; Kane, J.P. Fatty acid binding protein. Isolation from rat liver, characterization, and immunochemical quantification. J. Biol. Chem. 1982, 257, 7872–7878.
- Lowe, J.B.; Boguski, M.S.; Sweetser, D.A.; Elshourbagy, N.A.; Taylor, J.M.; Gordon, J.I. Human liver fatty acid binding protein. Isolation of a full length cDNA and comparative sequence analyses of orthologous and paralogous proteins. J. Biol. Chem. 1985, 260, 3413–3417.
- Sweetser, D.A.; Lowe, J.B.; Gordon, J.I. The nucleotide sequence of the rat liver fatty acid-binding protein gene. Evidence that exon 1 encodes an oligopeptide domain shared by a family of proteins which bind hydrophobic ligands. J. Biol. Chem. 1986, 261, 5553–5561.
- Alpers, D.H.; Strauss, A.W.; Ockner, R.K.; Bass, N.M.; Gordon, J.I. Cloning of a cDNA encoding rat intestinal fatty acid binding protein. Proc. Natl. Acad. Sci. USA 1984, 81, 313–317.
- Sweetser, D.A.; Birkenmeier, E.H.; Klisak, I.J.; Zollman, S.; Sparkes, R.S.; Mohandas, T.; Lusis, A.J.; Gordon, J.I. The human and rodent intestinal fatty acid binding protein genes. A comparative analysis of their structure, expression, and linkage relationships. J. Biol. Chem. 1987, 262, 16060–16071.
- Green, R.P.; Cohn, S.M.; Sacchettini, J.C.; Jackson, K.E.; Gordon, J.I. The mouse intestinal fatty acid binding protein gene: Nucleotide sequence, pattern of developmental and regional expression, and proposed structure of its protein product. DNA Cell Biol. 1992, 11, 31–41.
- Sacchettini, J.C.; Said, B.; Schulz, H.; Gordon, J.I. Rat heart fatty acid-binding protein is highly homologous to the murine adipocyte 422 protein and the P2 protein of peripheral nerve myelin. J. Biol. Chem. 1986, 261, 8218–8223.
- Heuckeroth, R.O.; Birkenmeier, E.H.; Levin, M.S.; Gordon, J.I. Analysis of the tissue-specific expression, developmental regulation, and linkage relationships of a rodent gene encoding heart fatty acid binding protein. J. Biol. Chem. 1987, 262, 9709–9717.
- Kurtz, A.; Zimmer, A.; Schnütgen, F.; Brüning, G.; Spener, F.; Müller, T. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development 1994, 120, 2637–2649.
- Sellner, P.A.; Chu, W.; Glatz, J.F.; Berman, N.E. Developmental role of fatty acid-binding proteins in mouse brain. Brain Res. Dev. Brain Res. 1995, 89, 33–46.
- Veerkamp, J.H.; Zimmerman, A.W. Fatty acid-binding proteins of nervous tissue. J. Mol. Neurosci. 2001, 16, 133–142; discussion 151–157.
- Spiegelman, B.M.; Frank, M.; Green, H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J. Biol. Chem. 1983, 258, 10083–10089.
- Bernlohr, D.A.; Angus, C.W.; Lane, M.D.; Bolanowski, M.A.; Kelly, T.J., Jr. Expression of specific mRNAs during adipose differentiation: Identification of an mRNA encoding a homologue of myelin P2 protein. Proc. Natl. Acad. Sci. USA 1984, 81, 5468–5472.
- Amri, E.Z.; Bertrand, B.; Ailhaud, G.; Grimaldi, P. Regulation of adipose cell differentiation. I. Fatty acids are inducers of the aP2 gene expression. J. Lipid Res. 1991, 32, 1449–1456.
- Bernlohr, D.A.; Coe, N.R.; Simpson, M.A.; Hertzel, A.V. Regulation of gene expression in adipose cells by polyunsaturated fatty acids. Adv. Exp. Med. Biol. 1997, 422, 145–156.
- Madsen, P.; Rasmussen, H.H.; Leffers, H.; Honoré, B.; Celis, J.E. Molecular cloning and expression of a novel keratinocyte protein (psoriasis-associated fatty acid-binding protein ) that is highly up-regulated in psoriatic skin and that shares similarity to fatty acid-binding proteins. J. Investig. Dermatol. 1992, 99, 299–305.
- Krieg, P.; Feil, S.; Fürstenberger, G.; Bowden, G.T. Tumor-specific overexpression of a novel keratinocyte lipid-binding protein. Identification and characterization of a cloned sequence activated during multistage carcinogenesis in mouse skin. J. Biol. Chem. 1993, 268, 17362–17369.
- Liu, Y.; Longo, L.D.; De Leon, M. In situ and immunocytochemical localization of E-FABP mRNA and protein during neuronal migration and differentiation in the rat brain. Brain Res. 2000, 852, 16–27.
- Gong, Y.Z.; Everett, E.T.; Schwartz, D.A.; Norris, J.S.; Wilson, F.A. Molecular cloning, tissue distribution, and expression of a 14-kDa bile acid-binding protein from rat ileal cytosol. Proc. Natl. Acad. Sci. USA 1994, 91, 4741–4745.
- Vodenlich, A.D., Jr.; Gong, Y.Z.; Geoghegan, K.F.; Lin, M.C.; Lanzetti, A.J.; Wilson, F.A. Identification of the 14 kDa bile acid transport protein of rat ileal cytosol as gastrotropin. Biochem. Biophys. Res. Commun. 1991, 177, 1147–1154.
- Walz, D.A.; Wider, M.D.; Snow, J.W.; Dass, C.; Desiderio, D.M. The complete amino acid sequence of porcine gastrotropin, an ileal protein which stimulates gastric acid and pepsinogen secretion. J. Biol. Chem. 1988, 263, 14189–14195.
- Gantz, I.; Nothwehr, S.F.; Lucey, M.; Sacchettini, J.C.; DelValle, J.; Banaszak, L.J.; Naud, M.; Gordon, J.I.; Yamada, T. Gastrotropin: Not an enterooxyntin but a member of a family of cytoplasmic hydrophobic ligand binding proteins. J. Biol. Chem. 1989, 264, 20248–20254.
- Feng, L.; Hatten, M.E.; Heintz, N. Brain lipid-binding protein (BLBP): A novel signaling system in the developing mammalian CNS. Neuron 1994, 12, 895–908.
- Shimizu, F.; Watanabe, T.K.; Shinomiya, H.; Nakamura, Y.; Fujiwara, T. Isolation and expression of a cDNA for human brain fatty acid-binding protein (B-FABP). Biochim. Biophys. Acta 1997, 1354, 24–28.
- Oko, R.; Morales, C.R. A novel testicular protein, with sequence similarities to a family of lipid binding proteins, is a major component of the rat sperm perinuclear theca. Dev. Biol. 1994, 166, 235–245.
- Pouresmaeili, F.; Morales, C.R.; Oko, R. Molecular cloning and structural analysis of the gene encoding PERF 15 protein present in the perinuclear theca of the rat spermatozoa. Biol. Reprod. 1997, 57, 655–659.
- de Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535.
- Hirsch, L.; Jette, N.; Frolkis, A.; Steeves, T.; Pringsheim, T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2016, 46, 292–300.
- Ruan, X.; Lin, F.; Wu, D.; Chen, L.; Weng, H.; Yu, J.; Wang, Y.; Chen, Y.; Chen, X.; Ye, Q.; et al. Comparative Efficacy and Safety of Dopamine Agonists in Advanced Parkinson’s Disease With Motor Fluctuations: A Systematic Review and Network Meta-Analysis of Double-Blind Randomized Controlled Trials. Front. Neurosci. 2021, 15, 728083.
- Parkinson, J. An essay on the shaking palsy. 1817. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 223–236; discussion 222.
- Phillips, O.; Ghosh, D.; Fernandez, H.H. Parkinson Disease Dementia Management: An Update of Current Evidence and Future Directions. Curr. Treat. Options Neurol. 2023, 25, 93–119.
- Takeda, A.; Baba, T.; Kikuchi, A.; Hasegawa, T.; Sugeno, N.; Konno, M.; Miura, E.; Mori, E. Olfactory dysfunction and dementia in Parkinson’s disease. J. Park. Dis. 2014, 4, 181–187.
- Takeda, A. How Useful is an Olfactory Test for Diagnosing Alzheimer’s Syndrome? Brain Nerve 2023, 75, 943–948.
- Rey, N.L.; Wesson, D.W.; Brundin, P. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol. Dis. 2018, 109, 226–248.
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840.
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473.
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Cairns, N.J.; Lantos, P.L.; Goedert, M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci. Lett. 1998, 251, 205–208.
- Shetty, A.S.; Bhatia, K.P.; Lang, A.E. Dystonia and Parkinson’s disease: What is the relationship? Neurobiol. Dis. 2019, 132, 104462.
- Montague, P.R.; Hyman, S.E.; Cohen, J.D. Computational roles for dopamine in behavioural control. Nature 2004, 431, 760–767.
- Schultz, W. Behavioral theories and the neurophysiology of reward. Annu. Rev. Psychol. 2006, 57, 87–115.
- Berridge, K.C. From prediction error to incentive salience: Mesolimbic computation of reward motivation. Eur. J. Neurosci. 2012, 35, 1124–1143.
- Stern, G. The effects of lesions in the substantia nigra. Brain 1966, 89, 449–478.
- Hoefer, P.F.A. Action Potentials of Muscles in Rigidity and Tremor. Arch. Neurol. Psychiatry 1940, 43, 704–725.
- Nagatsu, T. The catecholamine system in health and disease -Relation to tyrosine 3-monooxygenase and other catecholamine-synthesizing enzymes. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2007, 82, 388–415.
- Nagatsu, T.; Nagatsu, I. Tyrosine hydroxylase (TH), its cofactor tetrahydrobiopterin (BH4), other catecholamine-related enzymes, and their human genes in relation to the drug and gene therapies of Parkinson’s disease (PD): Historical overview and future prospects. J. Neural Transm. 2016, 123, 1255–1278.
- Nagatsu, T. Catecholamines and Parkinson’s disease: Tyrosine hydroxylase (TH) over tetrahydrobiopterin (BH4) and GTP cyclohydrolase I (GCH1) to cytokines, neuromelanin, and gene therapy: A historical overview. J. Neural Transm. 2023. ahead of print.
- Nagatsu, T.; Levitt, M.; Udenfriend, S. Tyrosine Hydroxylase. The initial step in norepinephrine biosynthesis. J. Biol. Chem. 1964, 239, 2910–2917.
- Fukui, N.; Yamamoto, H.; Miyabe, M.; Aoyama, Y.; Hongo, K.; Mizobata, T.; Kawahata, I.; Yabuki, Y.; Shinoda, Y.; Fukunaga, K.; et al. An alpha-synuclein decoy peptide prevents cytotoxic alpha-synuclein aggregation caused by fatty acid binding protein 3. J. Biol. Chem. 2021, 296, 100663.
- Wang, H.; Fukunaga, K.; Cheng, A.; Wang, Y.; Arimura, N.; Yoshino, H.; Sasaki, T.; Kawahata, I. Novel FABP3 ligand, HY-11-9, ameliorates neuropathological deficits in MPTP-induced Parkinsonism in mice. J. Pharmacol. Sci. 2023, 152, 30–38.
- Guo, Q.; Kawahata, I.; Jia, W.; Wang, H.; Cheng, A.; Yabuki, Y.; Shioda, N.; Fukunaga, K. alpha-Synuclein decoy peptide protects mice against alpha-synuclein-induced memory loss. CNS Neurosci. Ther. 2023, 29, 1547–1560.
- Yabuki, Y.; Matsuo, K.; Kawahata, I.; Fukui, N.; Mizobata, T.; Kawata, Y.; Owada, Y.; Shioda, N.; Fukunaga, K. Fatty Acid Binding Protein 3 Enhances the Spreading and Toxicity of alpha-Synuclein in Mouse Brain. Int. J. Mol. Sci. 2020, 21, 2230.
- Wang, Y.; Shinoda, Y.; Cheng, A.; Kawahata, I.; Fukunaga, K. Epidermal Fatty Acid-Binding Protein 5 (FABP5) Involvement in Alpha-Synuclein-Induced Mitochondrial Injury under Oxidative Stress. Biomedicines 2021, 9, 110.
- Cheng, A.; Jia, W.; Kawahata, I.; Fukunaga, K. A novel fatty acid-binding protein 5 and 7 inhibitor ameliorates oligodendrocyte injury in multiple sclerosis mouse models. EBioMedicine 2021, 72, 103582.
- Cheng, A.; Kawahata, I.; Fukunaga, K. Fatty Acid Binding Protein 5 Mediates Cell Death by Psychosine Exposure through Mitochondrial Macropores Formation in Oligodendrocytes. Biomedicines 2020, 8, 635.
- Guo, Q.; Kawahata, I.; Degawa, T.; Ikeda-Matsuo, Y.; Sun, M.; Han, F.; Fukunaga, K. Fatty Acid-Binding Proteins Aggravate Cerebral Ischemia-Reperfusion Injury in Mice. Biomedicines 2021, 9, 529.
- Guo, Q.; Kawahata, I.; Cheng, A.; Wang, H.; Jia, W.; Yoshino, H.; Fukunaga, K. Fatty acid-binding proteins 3 and 5 are involved in the initiation of mitochondrial damage in ischemic neurons. Redox Biol. 2023, 59, 102547.
- Cheng, A.; Wang, Y.F.; Shinoda, Y.; Kawahata, I.; Yamamoto, T.; Jia, W.B.; Yamamoto, H.; Mizobata, T.; Kawata, Y.; Fukunaga, K. Fatty acid-binding protein 7 triggers alpha-synuclein oligomerization in glial cells and oligodendrocytes associated with oxidative stress. Acta Pharmacol. Sin. 2022, 43, 552–562.
- Cheng, A.; Kawahata, I.; Wang, Y.; Jia, W.; Wang, H.; Sekimori, T.; Chen, Y.; Suzuki, H.; Takeda, A.; Stefanova, N.; et al. Epsin2, a novel target for multiple system atrophy therapy via alpha-synuclein/FABP7 propagation. Brain 2023, 146, 3172–3180.
- Cheng, A.; Jia, W.; Finkelstein, D.I.; Stefanova, N.; Wang, H.; Sasaki, T.; Kawahata, I.; Fukunaga, K. Pharmacological inhibition of FABP7 by MF 6 counteracts cerebellum dysfunction in an experimental multiple system atrophy mouse model. Acta Pharmacol. Sin. 2023. ahead of print.
- Ntetsika, T.; Papathoma, P.E.; Markaki, I. Novel targeted therapies for Parkinson’s disease. Mol. Med. 2021, 27, 17.
- Prasad, E.M.; Hung, S.Y. Current Therapies in Clinical Trials of Parkinson’s Disease: A 2021 Update. Pharmaceuticals 2021, 14, 717.
- Mollenhauer, B.; Cullen, V.; Kahn, I.; Krastins, B.; Outeiro, T.F.; Pepivani, I.; Ng, J.; Schulz-Schaeffer, W.; Kretzschmar, H.A.; McLean, P.J.; et al. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp. Neurol. 2008, 213, 315–325.
- Li, X.; Fan, X.; Yang, H.; Liu, Y. Review of Metabolomics-Based Biomarker Research for Parkinson’s Disease. Mol. Neurobiol. 2022, 59, 1041–1057.
- Ma, Z.L.; Wang, Z.L.; Zhang, F.Y.; Liu, H.X.; Mao, L.H.; Yuan, L. Biomarkers of Parkinson’s Disease: From Basic Research to Clinical Practice. Aging Dis. 2023. ahead of print.
- Tsamourgelis, A.; Swann, P.; Chouliaras, L.; O’Brien, J.T. From protein biomarkers to proteomics in dementia with Lewy Bodies. Ageing Res. Rev. 2023, 83, 101771.
- Baba, T.; Kikuchi, A.; Hirayama, K.; Nishio, Y.; Hosokai, Y.; Kanno, S.; Hasegawa, T.; Sugeno, N.; Konno, M.; Suzuki, K.; et al. Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson’s disease: A 3 year longitudinal study. Brain 2012, 135, 161–169.
- Domellof, M.E.; Lundin, K.F.; Edstrom, M.; Forsgren, L. Olfactory dysfunction and dementia in newly diagnosed patients with Parkinson’s disease. Park. Relat. Disord. 2017, 38, 41–47.
- Fullard, M.E.; Morley, J.F.; Duda, J.E. Olfactory Dysfunction as an Early Biomarker in Parkinson’s Disease. Neurosci. Bull. 2017, 33, 515–525.
- Chiasserini, D.; Biscetti, L.; Eusebi, P.; Salvadori, N.; Frattini, G.; Simoni, S.; De Roeck, N.; Tambasco, N.; Stoops, E.; Vanderstichele, H.; et al. Differential role of CSF fatty acid binding protein 3, alpha-synuclein, and Alzheimer’s disease core biomarkers in Lewy body disorders and Alzheimer’s dementia. Alzheimers Res. Ther. 2017, 9, 52.
- Jellinger, K.A.; Korczyn, A.D. Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med. 2018, 16, 34.
- Sepe, F.N.; Chiasserini, D.; Parnetti, L. Role of FABP3 as biomarker in Alzheimer’s disease and synucleinopathies. Future Neurol. 2018, 13, 199–207.
- Sezgin, M.; Bilgic, B.; Tinaz, S.; Emre, M. Parkinson’s Disease Dementia and Lewy Body Disease. Semin. Neurol. 2019, 39, 274–282.
- Walker, L.; Stefanis, L.; Attems, J. Clinical and neuropathological differences between Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies—Current issues and future directions. J. Neurochem. 2019, 150, 467–474.
- Parnetti, L.; Gaetani, L.; Eusebi, P.; Paciotti, S.; Hansson, O.; El-Agnaf, O.; Mollenhauer, B.; Blennow, K.; Calabresi, P. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 2019, 18, 573–586.
- Triozzi, P.L.; Stirling, E.R.; Song, Q.; Westwood, B.; Kooshki, M.; Forbes, M.E.; Holbrook, B.C.; Cook, K.L.; Alexander-Miller, M.A.; Miller, L.D.; et al. Circulating Immune Bioenergetic, Metabolic, and Genetic Signatures Predict Melanoma Patients’ Response to Anti-PD-1 Immune Checkpoint Blockade. Clin. Cancer Res. 2022, 28, 1192–1202.
- Lu, Z.; Priya Rajan, S.A.; Song, Q.; Zhao, Y.; Wan, M.; Aleman, J.; Skardal, A.; Bishop, C.; Atala, A.; Lu, B. 3D scaffold-free microlivers with drug metabolic function generated by lineage-reprogrammed hepatocytes from human fibroblasts. Biomaterials 2021, 269, 120668.
- Sinclair, E.; Trivedi, D.K.; Sarkar, D.; Walton-Doyle, C.; Milne, J.; Kunath, T.; Rijs, A.M.; de Bie, R.M.A.; Goodacre, R.; Silverdale, M.; et al. Metabolomics of sebum reveals lipid dysregulation in Parkinson’s disease. Nat. Commun. 2021, 12, 1592.
- Oizumi, H.; Sugimura, Y.; Totsune, T.; Kawasaki, I.; Ohshiro, S.; Baba, T.; Kimpara, T.; Sakuma, H.; Hasegawa, T.; Kawahata, I.; et al. Plasma sphingolipid abnormalities in neurodegenerative diseases. PLoS ONE 2022, 17, e0279315.
