Hepatocellular carcinoma (HCC) is one of the most frequent cancers in humans, characterised by a high resistance to conventional chemotherapy, late diagnosis, and a high mortality rate. It is necessary to elucidate the molecular mechanisms involved in hepatocarcinogenesis to improve diagnosis and treatment outcomes. The Runt-related (RUNX) family of transcription factors (RUNX1, RUNX2, and RUNX3) participates in cardinal biological processes and plays paramount roles in the pathogenesis of numerous human malignancies. Their role is often controversial as they can act as oncogenes or tumour suppressors and depends on cellular context. Evidence shows that deregulated RUNX genes may be involved in hepatocarcinogenesis from the earliest to the latest stages.
- RUNX
- hepatocellular carcinoma
- oncogenes
- tumour suppressors
- biomarkers
1. Introduction
1.1. RUNX Genes’ and Proteins’ Structure
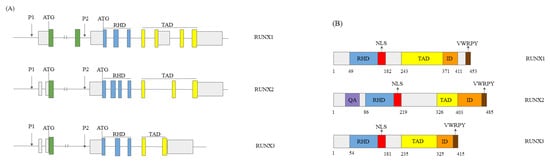
1.2. The Role of the RUNX Genes in Normal Development
RUNX genes engage in normal cell development and differentiation processes, playing a tissue-specific role [13][14]. RUNX1 is crucial for cell growth and differentiation of immune cells, epithelial stem and epithelial cells, and neurodevelopment [11][14][11,15]. RUNX1 acts in the development of hematopoietic cells in vertebrates [15][16][13,16]. Consequently, the chromosomal rearrangements and gene mutations involving RUNX1 lead to various leukaemia types [15][13]. RUNX2 is a factor in bone generation [11]. RUNX2 knockout mice lack osteoblast differentiation, leading to osteoporosis [17]. RUNX3 is essential for embryogenesis, and RUNX3 knockout mice die quickly [18].1.3. The Role of RUNX Genes in Cancer
RUNX genes’ mutations and abnormal expression lead to various cancer types. RUNX genes can hinder or activate tumourigenesis [12]. A recent study by Pan and colleagues [19][22] suggests that RUNX genes’ aberrant expression causes disparate cancer types and influences disease prognosis. RUNX1 plays a cardinal role in hematopoiesis and, consequently, in haematological tumours. The researchers documented mutations of the RUNX1 gene in acute myeloid leukaemia (AML) [20][23], acute lymphoid leukaemia (ALL), and familial platelet disorder with a predisposition to acute myeloid leukaemia (FPD/AML). These mutations either influence or do not influence the binding ability of RUNX1 to the other target genes, with the more or less changed function, depending on the mutated region (DBD, TAD, or nuclear localisation domain). RUNX1 expression changes are also found in solid tumours, like glioblastoma, ovarian, colon, breast, and hepatocellular carcinoma, where in tumour cells and available patient samples, it predominantly acts as a tumour suppressive. However, its oncogenic function is also documented [21][22][23][24][25][26][31,32,33,34,35,36]. RUNX2 is involved in the progression of various human tumours by regulating cell proliferation, angiogenesis, cancer stemness, and metastasis [27][37]. Its expression could be deregulated by several mechanisms. Recent research has uncovered numerous somatic mutations in the RUNX2 gene in various cancers, including missense, nonsense, and nonstop mutations and frameshift insertions and deletions [27][37]. RUNX3 is involved in carcinogenesis by interacting with several oncogenic signalling pathways, acting as a tumour suppressor in some tumours [28][29][30][31][32][40,41,42,43,44] and as an oncogene in others [33][34][35][45,46,47]. RUNX3 is frequently inactivated in human cancers by promoter DNA hypermethylation [28][29][30][31][32][36][37][38][39][40][41][40,41,42,43,44,48,49,50,51,52,53], histone modification [42][43][54,55], hemizygous deletion [38][39][50,51], and protein mislocalisation [6][31][6,43].1.4. The Mechanisms of Action of RUNX Proteins
Also, the co-expression of RUNX genes with epigenetic regulators could affect the onset of some cancer types. Researchers should pay attention to epigenetic mechanisms of RUNX genes’ regulation, including DNA methylation and miRNAs [19][22]. As epigenetic regulators, RUNX proteins cooperate with diverse coregulators and involve many signal transduction processes. In addition, they participate in chromatin landscape remodelling [10]. There is evidence that RUNX proteins can function as pioneer transcription factors that recruit various chromatin remodelling enzymes and other transcription factors to open the condensed chromatin structure and thus activate the transcription of target genes [10][19][44][10,22,65]. This epigenetic role of RUNX genes appears to play a pivotal role in both physiological and pathological conditions, including cancer [10][19][10,22].2. RUNX1 in HCC
2.1. RUNX1 Role in HCC
The role of RUNX1 in solid tumours is controversial, acting in two opposite ways. It can impede or promote carcinogenesis, as reviewed in [7][45][7,76]. Databases that collect transcriptomic studies and link data on the genomic and clinical parameters with various cancer groups show controversial results on the RUNX1 expression. According to the Gent2 and TIMER2.0 databases, there was a significantly higher expression of RUNX1 transcript in hepatocellular carcinoma compared to normal tissue [46][47][77,78]. However, the UCSC database shows the opposite results [48][79]. It has an upgraded version [49][80]. The RUNX1 expression was decreased in hepatocellular carcinoma. That was consistent with the work of Miyagawa and colleagues, who noticed that RUNX1 mRNA was 76% and 47% lower in HCC and cirrhotic tissue than in normal tissue. Also, there was a significant decrease in RUNX1 mRNA in HCC compared to cirrhotic liver samples [50][81]. One of the crucial processes in cancer progression is angiogenesis. In hematopoietic cell differentiation from hemogenic endothelium cells, RUNX1 has a vital role. The RUNX1-deficient mice lack hematopoiesis and angiogenesis [51][83]. Added exogenously, IGFBP-3 inhibited RUNX1-promoted angiogenesis dose-dependently [52][84]. Thus, it can conclude that RUNX1 has a cardinal role in angiogenic differentiation and vascularisation. Vascular endothelial growth factor (VEGF) is an angiogenesis modulator in a cancer cell environment and the negative prognostic factor for acute myeloid leukaemia. In the HCC cell culture, Elst and colleagues found that RUNX1 inhibited VEGF expression [53][85].2.2. RUNX1 and miRNAs
There are not many studies of epigenetic processes involving the RUNX1 gene. Tuo and colleagues noticed the hypomethylation of the RUNX1 promoter in hepatocellular carcinoma [54][86]. Some studies show the association between several miRNAs and RUNX1 in HCC. Transcript 1 of RUNX1 (RUNX1-IT1) is a long non-coding sequence of an RNA transcript of the RUNX1 gene [55][87]. Yan and colleagues noticed the RUNX1 expression decrease in the HCC patients’ samples, and the knocking-down of RUNX1-IT1 increased the proliferation and reduced apoptosis in HCC cells [56][88]. Sun and colleagues observed the association of decreased RUNX1-IT1 expression with shorter DFS and OS. RUNX1-IT1 binds mir-632, competing with the other RNAs in HCC cells for target gene GSK-3β binding and modulating the WNT/β-catenin signalling cascade. Added hypoxia-prompted histone deacetylase 3 (HDAC3) in HCC cells reduced the RUNX1-IT1 expression. They concluded that the goal of HCC therapy should be to activate RUNX1-IT1 [57][89]. On the other hand, Vivacqua and colleagues noticed that oestrogen receptor agonists, such as the G protein-coupled oestrogen receptor agonist (G-1) and 17β-oestradiol (E2), increased miR-144 expression in HepG2 hepatocarcinoma cells, via the G protein-coupled oestrogen receptor 1 (GPER) and the PI3K/ERK1/2/Elk1 pathway. miR-144 then downregulates RUNX1, promoting the cell cycle [58][90].In conclusion, RUNX1 binds target genes (VEGF and COL4A1) and involves signalling pathways of cancer proliferation, metastasis, and angiogenesis. RUNX1 also interacts with several miRNAs, for example, mir-632 and mir-144. Given the importance of RUNX1 in hepatocellular carcinoma, its potential suitability as a treatment target requires additional studies.
3. RUNX2 in HCC
3.1. General Role of RUNX2 in HCC
According to literature data, the expression of RUNX2 on mRNA and/or protein level is elevated in HCC cell lines, as well as in liver tumour tissue [59][60][61][95,103,104], suggesting that this transcription factor has a role in hepatocarcinogenesis. Previous findings confirmed higher RUNX2 expression in HCC patients than expression detected in non-tumour tissues or healthy controls [59][60][62][95,103,105]. Wang and colleagues noticed that increased expression of RUNX2 significantly correlates with unfavourable clinicopathological features in HCC. These adverse features included the onset of multiple tumour nodes, higher histological grades and TNM stages, and venous invasion presence [60][103].3.2. RUNX2 Tumour Invasion Activity in HCC
The general mechanisms underlying the role of RUNX2 in various tumour types provide directions for detailed studies on the impact of RUNX2 on the pathogenesis of HCC. Many reports showed that increased RUNX2 expression enhances tumour cell migration and invasive properties [63][64][65][66][67][68][69][106,107,108,109,110,111,112]. Previous studies revealed the crucial role of the RUNX2 in the regulation of the epithelial-to-mesenchymal transition (EMT) process in many tumours [61][70][104,113], which is the first step toward tumour invasion and metastatic potential (Figure 23).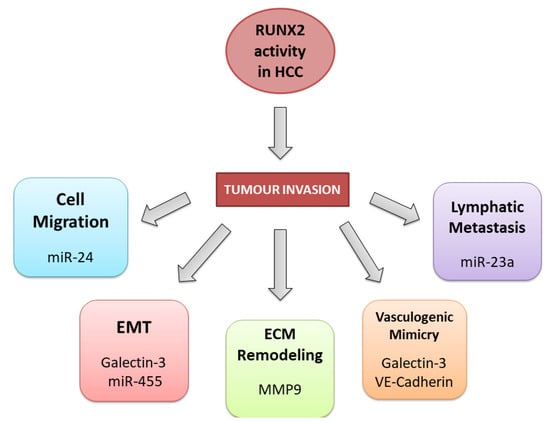
3.3. RUNX2 and Non-Coding RNAs in HCC
4. RUNX3 in HCC
4.1. General Role of RUNX3 in HCC
Several studies have shown that RUNX3 inactivation is cardinal for the initiation and progression of HCC [78][79][80][81][82][83][98,126,127,128,129,130]. As a multifunctional transcription factor, RUNX3 is implicated in diverse signalling pathways and cellular processes, thereby exerting multiple effects on tumour suppression [84][85][131,132]. According to current knowledge, RUNX3 participates in the regulation of the cell cycle [86][133], proliferation and apoptosis [87][134], angiogenesis [18], and EMT [78][88][98,135]. Its loss is also related to chemoresistance [89][90][136,137] (Figure 34).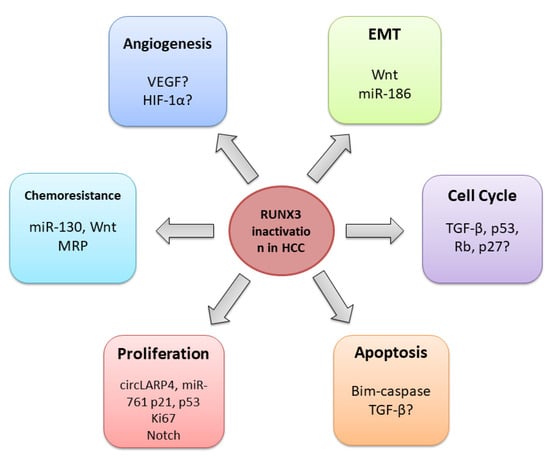
4.2. RUNX3 Regulates Cell Cycle, Proliferation, and Apoptosis
Dysregulation of the cell cycle is a prime event in hepatocarcinogenesis. RUNX3 may play a pivotal role in this process by employing diverse mechanisms [87][134]. Earlier studies on gastric epithelial cells demonstrated that RUNX3 regulates the cell cycle by interacting with p21, p27, and cyclin D1 proteins [91][92][93][138,139,140]. Further research revealed that RUNX3 induces the expression of the ARF and CDKN1A cell cycle regulators by interaction with BRD2 and pRB proteins [28][86][40,133]. As the major component of the transforming growth factor-beta signalling (TGF-β) pathway [8][94][8,141], RUNX3 can stop cell proliferation, inducing a p21 cell-cycle inhibitor [93][140]. Similarly, it can suppress apoptosis by inducing apoptosis initiator Bim, as has been shown on gastric cancer cell lines [95][142]. Another study in mice showed that proliferation marker Ki67 was more frequently observed in the RUNX3 knockout liver cells than in wild-type cells, which further confirmed the role of RUNX3 in hepatocyte proliferation regulation [18]. RUNX3 has been reported to control cellular senescence, a potent anti-cancer mechanism that prevents the proliferation of potentially cancerous cells [96][145]. Oncogenic activation of the Notch signalling pathway is also implicated in hepatocyte growth and proliferation [97][98][148,149]. Gao and colleagues have shown that RUNX3 can suppress oncogenic Notch signalling through direct interaction with the intracellular domain of the Notch1 protein in HCC cell lines [85][132].4.3. RUNX3 in the Angiogenesis Regulation
A crucial tumour-suppressive role of RUNX3 is angiogenesis prevention and tumour invasion. A recent study revealed that after the HCC therapeutic drug’s application, sorafenib, RUNX3 suppressed VEGF expression in HCC, which was associated with reduced tumour growth [99][151]. A previous study on gastric cancer cells demonstrated that RUNX3 destabilised hypoxia-inducible factor HIF-1α in the hypoxic microenvironment, thus inhibiting angiogenesis [100][152].4.4. RUNX3 and Epithelial-Mesenchymal Transition
Previous studies have shown that the loss of RUNX3 contributes to EMT, a crucial process related to metastasis, chemoresistance, and tumour stemness [101][102][154,155]. In vitro experiments demonstrated that RUNX3 repressed tumour metastasis and invasion by upregulating E-cadherin through the miR-186/E-cadherin/EMT axis [78][103][98,156].4.5. RUNX3 and Chemoresistance
A study on human HCC samples and cell lines demonstrated that RUNX3 could be downregulated by overexpression of miR-130 through the miR-130a/RUNX3/Wnt signalling pathway. This mechanism was associated with increased chemoresistance to cisplatin [90][137]. Studies in gastric [104][157] and cervical cancer [105][158] demonstrated that miR-130 directly binds to the RUNX3 and thus inhibits its expression. Accordingly, restoration of RUNX3 expression by targeting miR-130 could be a potential approach to overcome chemotherapy resistance in HCC patients.5. Conclusions
Despite considerable advances in cancer diagnosis and treatment, HCC remains one of the most common and hard-to-treat human cancers. Revealing the essential molecular processes underlying hepatocarcinogenesis is crucial for establishing reliable diagnostic, prognostic, and therapeutic markers. RUNX genes are often deregulated in HCC, exerting complex and conflicting functions. The role of RUNX1 is still contradictory, as there are reports of its tumour-suppressive but also oncogenic role in HCC. According to current knowledge, RUNX2 acts as an oncogene and is related to the more aggressive forms of the disease, whereas RUNX3 exerts a tumour-suppressive role and could be used as a biomarker for early HCC detection. All three genes could serve as therapeutic targets. However, a deeper understanding of the relationship between different RUNX family members and the signalling pathways they are involved in, considering the cell-specific microenvironment, is necessary for effective HCC therapeutic strategy development.
Increasing evidence suggests that RUNX genes act as epigenetic modulators that interact with other chromatin landscape regulators to activate or repress the transcription of target genes [89][136]. Since normal epigenetic patterns are altered in all types of human cancers, it would be of great interest to investigate interactions between RUNX proteins and other epigenetic regulators, especially in HCC. This could potentially provide an avenue for epigenetic therapy.
