Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Li Lin.
Epigenetic methylation has been shown to play an important role in transcriptional regulation and disease pathogenesis. Recent advancements in detection techniques have identified DNA N6-methyldeoxyadenosine (6mA) and RNA N6-methyladenosine (m6A) as methylation modifications at the sixth position of adenine in DNA and RNA, respectively.
- methylation
- N6-methyldeoxyadenosine
- N6-methyladenosine
1. Introduction
Epigenetics aims to explore the molecular mechanisms for stable genetic modifications of gene expression, protein functions, and ultimately cell fate without altering the DNA sequence, including DNA modification, RNA modification, histone modification, and non-coding RNA. These mechanisms regulate gene expression and chromatin structure. Recent studies have revealed the methylation on N-6 of adenine is a novel epigenetic modification that can be found at adenosine of DNA (N6-methyldeoxyadenosine, 6mA) and RNA (N6-methyladenosine, m6A). Both 6mA and m6A are present in mammalian cells, and notably, the m6A is abundant in the brain than in other mammalian tissues [1]. For DNA methylation, 5-methylcytosine (5mC) at CpG dinucleotides is the most abundant modified DNA base [2,3,4,5][2][3][4][5]. In addition, 5-hydroxymethylcytosine (5hmC) is not only an intermediate product of DNA demethylation but also a novel stable epigenetic regulator. Imbalances in the 5hmC levels contribute to neurological diseases, including neurodevelopmental and neurodegenerative diseases [6]. Similarly, 6mA, as a novel epigenetic modification on the DNA level, is highly enriched in the adult brain, regulates learning gene expression, and is associated with fear formation [7]. However, several studies have shown that the abundance DNA 6mA in the mammalian and even the human genome is very low [6]. A currently controversial issue is the presence of DNA-6mA in mammalian genomes [8,9][8][9].
Different RNA modifications have been reported on messenger RNA (mRNA), transfer RNA (tRNA), ribosomal RNA (rRNA), microRNA (miRNA), small nuclear RNA (snRNA), and long non-coding RNA (lncRNA) [10,11,12][10][11][12]. m6A is the most abundant in eukaryotic cells. Substantial evidence indicated that m6A was enriched in mammalian brains and embryonic stem cells (ESCs) and that its abundance increased continuously from the embryonic to the adult brain [1,13][1][13]. These results implicated that m6A may be involved in brain development, functional regulation, and synaptic plasticity. It has been demonstrated that m6A methylation affected neural stem cells, learning and memory, brain development, axon growth, and glioblastoma [14]. Abnormal m6A may cause neurological development and mental illness [15].
DNA 6mA has been reported early in prokaryotes and was not identified in eukaryotes until the last decade. Genome-wide profiling implicated a role for 6mA in regulating gene expression [16]. In contrast, RNA m6A methylation was studied earlier and widely. Increasing evidence uncovered the important role of m6A in human cancer progression and tumorigenesis [17]. In recent years, numerous studies have reported the genomic distribution and dynamics of 6mA and m6A in mammals, as well as their underlying functions in the regulation of gene expression. The higher abundance of 6mA and m6A in the mammalian brain sparked our interests in understanding their potential role in the central nervous system (CNS), specifically in development- and aging-related diseases. Current studies have reported a potential relationship between m6A and aging and neurodegenerative diseases; however, little is known about the role of 6mA. Interestingly, the methyl group is added to the same position of “A” at both DNA and RNA.
2. DNA 6mA Methylation and Its Writers, Erasers, and Readers
DNA 6mA was first discovered in prokaryotes and it widely exists in many species, which involves in the regulation of DNA replication, repair, transcription, transposition, and cell defense. It is produced by the addition of the methyl group from S-adenosyl-l-methionine (SAM) to N-6 of the adenine ring via specific methyltransferases [3]. In recent years, it has been reported that 6mA exists in eukaryotes, such as green algae [18], C. elegans [19], and Drosophila [20], but 6mA has been proved to exist at extremely low levels in most higher eukaryotes, especially in mammals. DNA 6mA modification is a dynamic process including the addition and removal of methyl groups, coordinated via a complex system of methylases. These proteins are called writers and erasers. In eukaryotes, several methyltransferases have been reported (Figure 1) [21]. DNA N6 adenine methyltransferase 1 (DAMT-1) has been identified in C. elegans to methylate 6mA via the knock down and overexpression of treatments. The DNA methyltransferases (DNMTs) family is known to act as 5mC methyltransferases in animals [22]. The overexpression or depletion of DNMT1 lead to the increased or decreased levels of genomic 6mA [23]. This suggested that DNMT1 may be a 6mA methyltransferase [24]. Further analysis revealed that DNMT1 belongs to the MT-A70 domain-containing enzyme family which includes mRNA methyltransferases Ime4 and Kar4 in yeast, and the methyltransferase-like protein 3 (METTL3) and METL14 in humans [25,26,27][25][26][27]. In mammals, METTL4 is a DNMT1 homologue and a paralogue of METTL3 and the methyltransferase-like protein14 (METTL14), which functions as a DNA 6mA methyltransferase. In the human genome, N-6 adenine-specific DNA methyltransferase 1 (N6AMT1) has reported as a methyltransferase, which contains the catalytic conserved motif NPPY [28]. This conclusion has been demonstrated via structural analysis and silence/overexpression.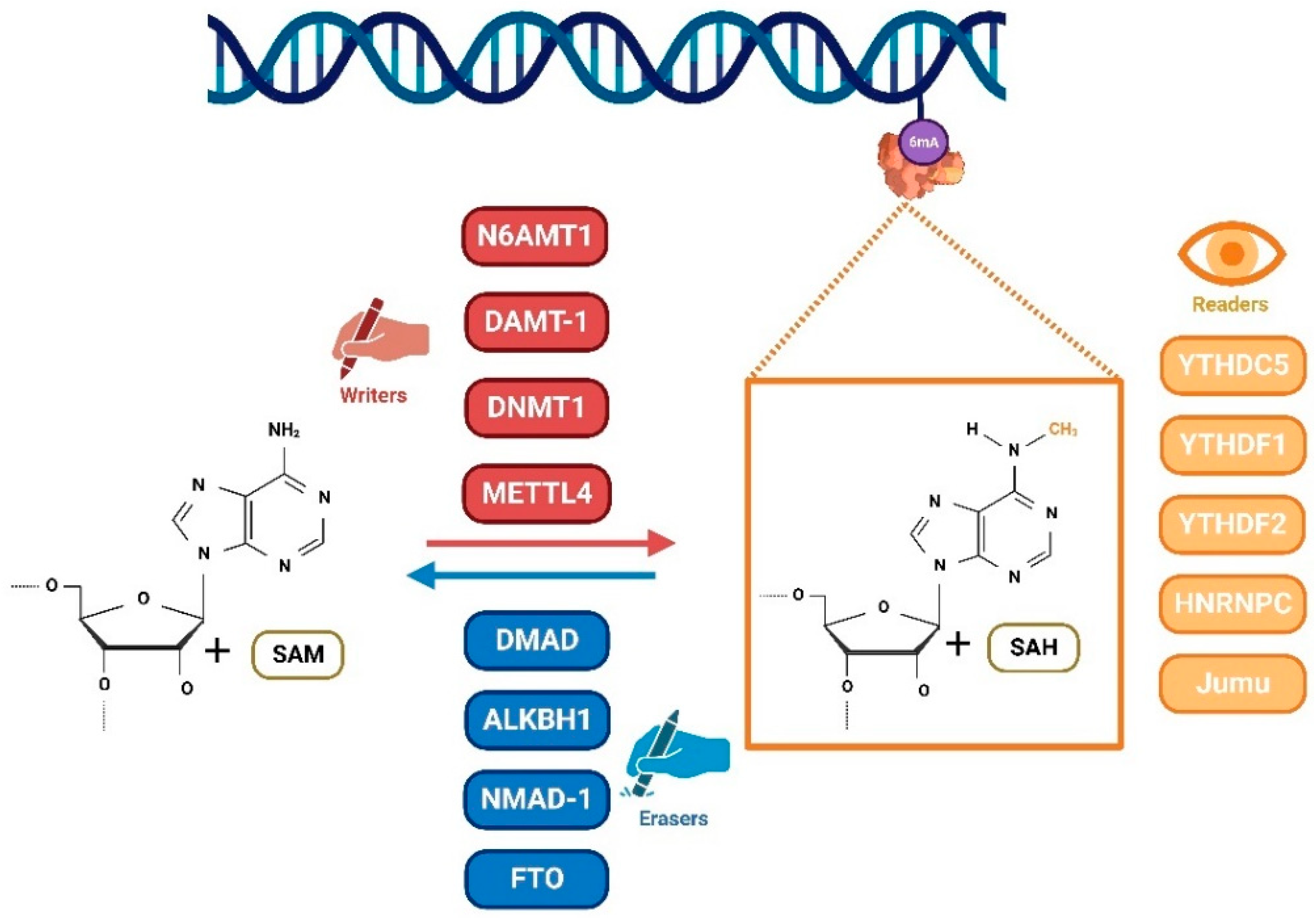
Figure 1. DNA 6mA modification is a dynamic process. Methyltransferases (writers), including N6AMT1, DAMT-1, DNMT1, and METTL4 add a methyl group to form 6mA. This process can be reversed via demethylases (erasers) such as DMAD, ALKBH1, NMAD-1, and FTO. In addition, binding proteins (readers) recognize and bind the 6mA site on DNA. Therefore, they also play an important role in the regulation of gene expression and cellular functions.
3. The Potential Functions of DNA 6mA Methylation
The 6mA modification has been well described in prokaryotes to play roles in foreign DNA cleavage, virus defense, and DNA damage repair [38,39,40,41][38][39][40][41]. The potential function of 6mA modification in mammals remains an active area of ongoing research. In addition to providing binding sites for effector proteins, one potential effect of the 6mA modification was to directly alter the overall structure of DNA. Early crystal structures suggested that 6mA may also alter DNA secondary structure [42]. Meanwhile, 6mA affected DNA double helix formation by altering the base pair stability and base stacking [40,42][40][42]. Similar to the main function of 5mC, 6mA modification has been found to play a role in regulating gene expression in different species [43]. The 6mA modification reduced the thermal stability of the DNA chain, which in turn changed the curvature of the DNA chain; thus, 6mA at a specific position affected the interaction of transcription factor-related elements [44]. 6mA modification acted as an epigenetic mark that affected the chromatin structure and gene expression patterns in a heritable manner. Recent studies showed that the coordination of 6mA and histone modification H3K4me2 contributed to the transgenerational epigenetic control [19], and 6mA was mainly present at ApT dinucleotides around the transcription start site (TSS) with a bimodal distribution and appeared to mark the active genes in Chlamydomonas. Furthermore, a genome-wide map of 6mA and its genomic distribution suggested a potential epigenetic role for 6mA in regulating gene expression [18]. Together, these findings suggested 6mA functions as an epigenetic mark in eukaryotes. In mouse embryonic stem cells, 6mA was associated with the repression and silencing of genes, particularly those on the X chromosome, and was known to play an important role in cell fate decisions. During the first 120 h of zebrafish embryonic development, the 6mA levels rose steadily from a pluripotent cell to a nearly fully formed individual, and the same pattern was observed in mouse embryonic days 7–21 [45]. With the continuous improvement of detection technology, 6mA modification has been detected in humans and is confirmed to be related to various diseases. It has been reported that 6mA was involved in the pathogenesis of essential hypertension [46]. The 6mA levels in leukocytes of all hypertension models were significantly reduced and could return to normal levels after successful hypertension treatment. The decreased 6mA levels in leukocytes and vascular smooth muscle cells (VSMCs) of the hypertensive models in vivo and in vitro were due to the increased ALKBH1 content. The 6mA was a sensitive marker for the development, diagnosis, and treatment of hypertension [46]. Moreover, 6mA was involved in the regulation of fatty liver degeneration. In patients with fatty liver degeneration, significantly increased the 6mA levels and downregulated ALKBH1 have been found [47]. Integrative analysis of transcriptome and chromatin immunoprecipitation-sequencing revealed that ALKBH1 directly bound and specifically demethylated the 6mA of genes involved in fatty acid uptake and lipogenesis, thereby reducing hepatic lipid accumulation [47]. Importantly, ALKBH1 overexpression was sufficient to suppress lipid uptake and synthesis and attenuate diet-induced hepatic steatosis and insulin resistance. ALKBH1-induced 6mA played an integral role in hepatic fatty acid metabolism as an epigenetic repressor and provided a potential therapeutic target for treatment. Aberrant DNA methylation has been shown to be associated with tumorigenesis. Recent reports indicated that 6mA participated in the progression and tumorigenesis of several cancers, such as breast cancer [48], gastric cancer [49], liver cancer [28], lung cancer, [50] and glioma [32]. Reduction in N6AMT1 in clinical breast cancer tissues correlated with 6mA intensity and predicted poorer survival of patients [48]. Functionally, the knockdown of N6AMT1 significantly decreased the 6mA levels in DNA and promoted colony formation and the migration of breast cancer cells, whereas the overexpression of N6AMT1 had the opposite effect. Mechanistically, N6AMT1 acted as a methyltransferase for 6mA formation and repressed the gene expression of key cell cycle inhibitors such as RB1 and TP53, which was also the first study on the regulation and function of 6mA modification in breast cancer progression and prognosis. The 6mA levels were apparently tissue specific, with the highest 6mA levels observed in the brain. It is well known that environmental exposure may induce epigenetic changes. What need to be pointed out here is that environmental factors in vivo and in vitro are inseparable from the expression level of 6mA. Yao et al. demonstrated that 6mA in the mouse brain was a dynamic change in response to environmental stress, and the change in 6mA was related to the expression of neuronal genes and LINE transposon. Stress-induced 6mA changes significantly related to genes which are associated with neuropsychiatric disorders. These results implicated that 6mA may play a key epigenetic role in mammalian brain development and pathogenesis [51]. Substantial evidence showed that physiological and psychological stress can alter DNA methylation of key stress-related genes in the mouse brain [52,53][52][53]. The prefrontal cortex (PFC), responsible for the highest-order cognitive abilities, is particularly vulnerable to chronic stress and plays an important role in depression. It has also been reported that after characterizing the dynamics of 6mA under starvation in the single-cell model organism, Tetrahymena thermophila, single-molecule real-time sequencing (SMRT-sequencing) showed that the 6mA levels in starved cells were significantly lower compared with the DNA 6mA levels in vegetatively growing cells [54]. In summary, environmental factors are extremely important for the influence of 6mA in different model organisms. 6mA is emerging as a crucial DNA epigenetic mark to regulate gene expression in eukaryotes. Current researchers explored the role of 6mA dysregulation and 6mA modulators in human diseases, especially in cancers [55]. However, the study of 6mA in mammals is still in its infancy, so further studies are needed to confirm the characterization of 6mA in mammals and expand ourthe knowledge of the biological functions of 6mA in human diseases, especially the physiological significance of its high abundance in the mammalian brain.References
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive Analysis of MRNA Methylation Reveals Enrichment in 3′ UTRs and near Stop Codons. Cell 2012, 149, 1635–1646.
- Edwards, J.R.; Yarychkivska, O.; Boulard, M.; Bestor, T.H. DNA Methylation and DNA Methyltransferases. Epigenet. Chromatin 2017, 10, 23.
- Breiling, A.; Lyko, F. Epigenetic Regulatory Functions of DNA Modifications: 5-Methylcytosine and Beyond. Epigenet. Chromatin 2015, 8, 24.
- Kumar, S.; Chinnusamy, V.; Mohapatra, T. Epigenetics of Modified DNA Bases: 5-Methylcytosine and Beyond. Front. Genet. 2018, 9, 640.
- Jones, P.A. Functions of DNA Methylation: Islands, Start Sites, Gene Bodies and Beyond. Nat. Rev. Genet. 2012, 13, 484–492.
- Koziol, M.J.; Bradshaw, C.R.; Allen, G.E.; Costa, A.S.H.; Frezza, C.; Gurdon, J.B. Identification of Methylated Deoxyadenosines in Vertebrates Reveals Diversity in DNA Modifications. Nat. Struct. Mol. Biol. 2016, 23, 24–30.
- Li, X.; Zhao, Q.; Wei, W.; Lin, Q.; Magnan, C.; Emami, M.R.; Wearick-Silva, L.E.; Viola, T.W.; Marshall, P.R.; Yin, J.; et al. The DNA Modification N6-Methyl-2′-Deoxyadenosine (M6dA) Drives Activity-Induced Gene Expression and Is Required for Fear Extinction. Nat. Neurosci. 2019, 22, 534–544.
- Douvlataniotis, K.; Bensberg, M.; Lentini, A.; Gylemo, B.; Nestor, C.E. No Evidence for DNA N 6-Methyladenine in Mammals. Sci. Adv. 2020, 6, eaay3335.
- Feng, X.; He, C. Mammalian DNA N6-Methyladenosine: Challenges and New Insights. Mol. Cell 2023, 83, 343–351.
- Motorin, Y.; Helm, M. RNA Nucleotide Methylation. Wiley Interdiscip. Rev. RNA 2011, 2, 611–631.
- Boccaletto, P.; Machnicka, M.A.; Purta, E.; Piatkowski, P.; Baginski, B.; Wirecki, T.K.; de Crécy-Lagard, V.; Ross, R.; Limbach, P.A.; Kotter, A.; et al. MODOMICS: A Database of RNA Modification Pathways. 2017 Update. Nucleic Acids. Res. 2018, 46, D303–D307.
- Cao, G.; Li, H.-B.; Yin, Z.; Flavell, R.A. Recent Advances in Dynamic M6A RNA Modification. Open Biol. 2016, 6, 160003.
- Chang, M.; Lv, H.; Zhang, W.; Ma, C.; He, X.; Zhao, S.; Zhang, Z.-W.; Zeng, Y.-X.; Song, S.; Niu, Y.; et al. Region-Specific RNA M6A Methylation Represents a New Layer of Control in the Gene Regulatory Network in the Mouse Brain. Open Biol. 2017, 7, 170166.
- Li, J.; Yang, X.; Qi, Z.; Sang, Y.; Liu, Y.; Xu, B.; Liu, W.; Xu, Z.; Deng, Y. The Role of MRNA M6A Methylation in the Nervous System. Cell Biosci. 2019, 9, 66.
- Widagdo, J.; Anggono, V. The M6A-Epitranscriptomic Signature in Neurobiology: From Neurodevelopment to Brain Plasticity. J. Neurochem. 2018, 147, 137–152.
- Gz, L.; He, C. DNA N6-Methyladenine in Metazoans: Functional Epigenetic Mark or Bystander? Nat. Struct. Mol. Biol. 2017, 24, 503–506.
- An, Y.; Duan, H. The Role of M6A RNA Methylation in Cancer Metabolism. Mol. Cancer 2022, 21, 14.
- Fu, Y.; Luo, G.-Z.; Chen, K.; Deng, X.; Yu, M.; Han, D.; Hao, Z.; Liu, J.; Lu, X.; Dore, L.C.; et al. N6-Methyldeoxyadenosine Marks Active Transcription Start Sites in Chlamydomonas. Cell 2015, 161, 879–892.
- Greer, E.L.; Blanco, M.A.; Gu, L.; Sendinc, E.; Liu, J.; Aristizábal-Corrales, D.; Hsu, C.-H.; Aravind, L.; He, C.; Shi, Y. DNA Methylation on N6-Adenine in C. Elegans. Cell 2015, 161, 868–878.
- Zhang, G.; Huang, H.; Liu, D.; Cheng, Y.; Liu, X.; Zhang, W.; Yin, R.; Zhang, D.; Zhang, P.; Liu, J.; et al. N6-Methyladenine DNA Modification in Drosophila. Cell 2015, 161, 893–906.
- Liang, Z.; Riaz, A.; Chachar, S.; Ding, Y.; Du, H.; Gu, X. Epigenetic Modifications of MRNA and DNA in Plants. Mol. Plant 2020, 13, 14–30.
- Song, J.; Rechkoblit, O.; Bestor, T.H.; Patel, D.J. Structure of DNMT1-DNA Complex Reveals a Role for Autoinhibition in Maintenance DNA Methylation. Science 2011, 331, 1036–1040.
- Zhang, H.; Gao, Q.; Tan, S.; You, J.; Lyu, C.; Zhang, Y.; Han, M.; Chen, Z.; Li, J.; Wang, H.; et al. SET8 Prevents Excessive DNA Methylation by Methylation-Mediated Degradation of UHRF1 and DNMT1. Nucleic Acids Res. 2019, 47, 9053–9068.
- Haggerty, C.; Kretzmer, H.; Riemenschneider, C.; Kumar, A.S.; Mattei, A.L.; Bailly, N.; Gottfreund, J.; Giesselmann, P.; Weigert, R.; Brändl, B.; et al. Dnmt1 Has de Novo Activity Targeted to Transposable Elements. Nat. Struct. Mol. Biol. 2021, 28, 594–603.
- Liu, J.; Yue, Y.; Han, D.; Wang, X.; Fu, Y.; Zhang, L.; Jia, G.; Yu, M.; Lu, Z.; Deng, X.; et al. A METTL3-METTL14 Complex Mediates Mammalian Nuclear RNA N6-Adenosine Methylation. Nat. Chem. Biol. 2014, 10, 93–95.
- Iyer, L.M.; Abhiman, S.; Aravind, L. Natural History of Eukaryotic DNA Methylation Systems. Prog. Mol. Biol. Transl. Sci. 2011, 101, 25–104.
- Bokar, J.A.; Shambaugh, M.E.; Polayes, D.; Matera, A.G.; Rottman, F.M. Purification and CDNA Cloning of the AdoMet-Binding Subunit of the Human MRNA (N6-Adenosine)-Methyltransferase. RNA 1997, 3, 1233–1247.
- Xiao, C.-L.; Zhu, S.; He, M.; Chen, D.; Zhang, Q.; Chen, Y.; Yu, G.; Liu, J.; Xie, S.-Q.; Luo, F.; et al. N6-Methyladenine DNA Modification in the Human Genome. Mol. Cell 2018, 71, 306–318.e7.
- Yao, B.; Li, Y.; Wang, Z.; Chen, L.; Poidevin, M.; Zhang, C.; Lin, L.; Wang, F.; Bao, H.; Jiao, B.; et al. Active N6-Methyladenine Demethylation by DMAD Regulates Gene Expression by Coordinating with Polycomb Protein in Neurons. Mol. Cell 2018, 71, 848–857.e6.
- Wang, S.Y.; Mao, H.; Shibuya, H.; Uzawa, S.; O’Brown, Z.K.; Wesenberg, S.; Shin, N.; Saito, T.T.; Gao, J.; Meyer, B.J.; et al. The Demethylase NMAD-1 Regulates DNA Replication and Repair in the Caenorhabditis Elegans Germline. PLoS Genet. 2019, 15, e1008252.
- Wu, T.P.; Wang, T.; Seetin, M.G.; Lai, Y.; Zhu, S.; Lin, K.; Liu, Y.; Byrum, S.D.; Mackintosh, S.G.; Zhong, M.; et al. DNA Methylation on N(6)-Adenine in Mammalian Embryonic Stem Cells. Nature 2016, 532, 329–333.
- Xie, Q.; Wu, T.P.; Gimple, R.C.; Li, Z.; Prager, B.C.; Wu, Q.; Yu, Y.; Wang, P.; Wang, Y.; Gorkin, D.U.; et al. N6-Methyladenine DNA Modification in Glioblastoma. Cell 2018, 175, 1228–1243.e20.
- Li, H.; Zhang, N.; Wang, Y.; Xia, S.; Zhu, Y.; Xing, C.; Tian, X.; Du, Y. DNA N6-Methyladenine Modification in Eukaryotic Genome. Front. Genet. 2022, 13, 914404.
- Nettersheim, D.; Berger, D.; Jostes, S.; Kristiansen, G.; Lochnit, G.; Schorle, H. N6-Methyladenosine Detected in RNA of Testicular Germ Cell Tumors Is Controlled by METTL3, ALKBH5, YTHDC1/F1/F2, and HNRNPC as Writers, Erasers, and Readers. Andrology 2019, 7, 498–506.
- Koh, C.W.Q.; Goh, Y.T.; Toh, J.D.W.; Neo, S.P.; Ng, S.B.; Gunaratne, J.; Gao, Y.-G.; Quake, S.R.; Burkholder, W.F.; Goh, W.S.S. Single-Nucleotide-Resolution Sequencing of Human N6-Methyldeoxyadenosine Reveals Strand-Asymmetric Clusters Associated with SSBP1 on the Mitochondrial Genome. Nucleic Acids Res. 2018, 46, 11659–11670.
- Shen, C.; Wang, K.; Deng, X.; Chen, J. DNA N6-Methyldeoxyadenosine in Mammals and Human Disease. Trends Genet. 2022, 38, 454–467.
- He, S.; Zhang, G.; Wang, J.; Gao, Y.; Sun, R.; Cao, Z.; Chen, Z.; Zheng, X.; Yuan, J.; Luo, Y.; et al. 6mA-DNA-Binding Factor Jumu Controls Maternal-to-Zygotic Transition Upstream of Zelda. Nat. Commun. 2019, 10, 2219.
- Cui, H.; Rong, W.; Ma, J.; Zhu, Q.; Jiang, B.; Zhang, L.; Li, C.; Zhuo, Z.; Chen, M. DNA N6-Adenine Methylation in HBV-Related Hepatocellular Carcinoma. Gene 2022, 822, 146353.
- Marinus, M.G.; Morris, N.R. Biological Function for 6-Methyladenine Residues in the DNA of Escherichia Coli K12. J. Mol. Biol. 1974, 85, 309–322.
- Boulias, K.; Greer, E.L. Means, Mechanisms and Consequences of Adenine Methylation in DNA. Nat. Rev. Genet. 2022, 23, 411–428.
- Pukkila, P.J.; Peterson, J.; Herman, G.; Modrich, P.; Meselson, M. Effects of High Levels of DNA Adenine Methylation on Methyl-Directed Mismatch Repair in Escherichia Coli. Genetics 1983, 104, 571–582.
- Sternglanz, H.; Bugg, C.E. Conformation of N6-Methyladenine, a Base Involved in DNA Modification: Restriction Processes. Science 1973, 182, 833–834.
- Mondo, S.J.; Dannebaum, R.O.; Kuo, R.C.; Louie, K.B.; Bewick, A.J.; LaButti, K.; Haridas, S.; Kuo, A.; Salamov, A.; Ahrendt, S.R.; et al. Widespread Adenine N6-Methylation of Active Genes in Fungi. Nat. Genet. 2017, 49, 964–968.
- Marinus, M.G.; Casadesus, J. Roles of DNA Adenine Methylation in Host-Pathogen Interactions: Mismatch Repair, Transcriptional Regulation, and More. FEMS Microbiol. Rev. 2009, 33, 488–503.
- Fernandes, S.B.; Grova, N.; Roth, S.; Duca, R.C.; Godderis, L.; Guebels, P.; Mériaux, S.B.; Lumley, A.I.; Bouillaud-Kremarik, P.; Ernens, I.; et al. N6-Methyladenine in Eukaryotic DNA: Tissue Distribution, Early Embryo Development, and Neuronal Toxicity. Front. Genet. 2021, 12, 657171.
- Guo, Y.; Pei, Y.; Li, K.; Cui, W.; Zhang, D. DNA N6-Methyladenine Modification in Hypertension. Aging 2020, 12, 6276–6291.
- Luo, L.; Liu, Y.; Nizigiyimana, P.; Ye, M.; Xiao, Y.; Guo, Q.; Su, T.; Luo, X.; Huang, Y.; Zhou, H. DNA 6mA Demethylase ALKBH1 Orchestrates Fatty Acid Metabolism and Suppresses Diet-Induced Hepatic Steatosis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 1213–1233.
- Chen, J.; Zhuang, Y.; Wang, P.; Ning, J.; Liu, W.; Huang, Y.; Lin, X.; Peng, L.; Zhang, D. Reducing N6AMT1-Mediated 6mA DNA Modification Promotes Breast Tumor Progression via Transcriptional Repressing Cell Cycle Inhibitors. Cell Death Dis. 2022, 13, 216.
- Wang, X.; Wong, C.C.; Chen, H.; Fu, K.; Shi, L.; Su, H.; Guo, S.; Gou, H.; Hu, X.; Zhang, L.; et al. The N6-Methyladenine DNA Demethylase ALKBH1 Promotes Gastric Carcinogenesis by Disrupting NRF1 Binding Capacity. Cell Rep. 2023, 42, 112279.
- Xiong, J.; Ye, T.-T.; Ma, C.-J.; Cheng, Q.-Y.; Yuan, B.-F.; Feng, Y.-Q. N 6-Hydroxymethyladenine: A Hydroxylation Derivative of N6-Methyladenine in Genomic DNA of Mammals. Nucleic Acids Res. 2019, 47, 1268–1277.
- Yao, B.; Cheng, Y.; Wang, Z.; Li, Y.; Chen, L.; Huang, L.; Zhang, W.; Chen, D.; Wu, H.; Tang, B.; et al. DNA N6-Methyladenine Is Dynamically Regulated in the Mouse Brain Following Environmental Stress. Nat. Commun. 2017, 8, 1122.
- Uchida, S.; Hara, K.; Kobayashi, A.; Otsuki, K.; Yamagata, H.; Hobara, T.; Suzuki, T.; Miyata, N.; Watanabe, Y. Epigenetic Status of Gdnf in the Ventral Striatum Determines Susceptibility and Adaptation to Daily Stressful Events. Neuron 2011, 69, 359–372.
- Elliott, E.; Manashirov, S.; Zwang, R.; Gil, S.; Tsoory, M.; Shemesh, Y.; Chen, A. Dnmt3a in the Medial Prefrontal Cortex Regulates Anxiety-Like Behavior in Adult Mice. J. Neurosci. 2016, 36, 730–740.
- Sheng, Y.; Pan, B.; Wei, F.; Wang, Y.; Gao, S. Case Study of the Response of N6-Methyladenine DNA Modification to Environmental Stressors in the Unicellular Eukaryote Tetrahymena Thermophila. mSphere 2021, 6, e0120820.
- Wu, K.-J. The Epigenetic Roles of DNA N6-Methyladenine (6mA) Modification in Eukaryotes. Cancer Lett. 2020, 494, 40–46.
More
