Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Alberto Dias.
Genista tridentata (L.) Willk., known as “prickled broom”, is a Leguminosae (Fabaceae) species native to the Iberian Peninsula, Morocco, Algeria, and Tunisia. It is used in folk medicine as an anti-inflammatory, for gastrointestinal and respiratory disorders, rheumatism, and headaches, to lower blood pressure, against hypercholesterolemia and hyperglycemia.
- traditional medicine
- biological properties
- Genista tridentata
- Pterospartum tridentatum
- nutraceuticals
1. Introduction
Wild edible plants are an important piece of the cultural and genetic heritage of distinct world regions, representing high importance, predominantly in rural and suburban areas [1]. Furthermore, they are interesting sources of bioactive compounds and need recognition as considerable contributors to human health promotion and disease prevention [2].
Genista tridentata (L.) Willk. (the recognized name for this species), also known as Pterospartum tridentatum (L.) Willk. (the commonly used name in both scientific literature and commercially available extracts. Among other synonyms, Chamaespartum tridentatum (P.) Gibbs is also used [3,4][3][4]). Commonly known as “prickled broom”, it is a Leguminosae (Fabaceae) species belonging to the subfamily Papilionoideae [5,6][5][6]. In line with scientific literature and the Global Biodiversity Information Facility database [7], the recorded countries of origin for the plant remain consistent, comprising Portugal, Spain, and Morocco. However, it is important to mention that the Plants of the World Online (POWO) database [8] also lists Algeria and Tunisia as potential countries of origin for this plant. This shrub can be found in the understory of Arbutus unedo, Pinus, and Eucalyptus forests, as well as in abandoned lands. It grows spontaneously up to 100 cm in acidic soils [9] and presents yellow flowers with a typical odor in alternate branches and coriaceous winged stems [10]. Traditionally, it is harvested in the spring between March and June.
G. tridentata is an aromatic plant that is very important in Portuguese gastronomy. The leaves are conventionally used as a condiment/spice for the seasoning of traditional rice and meat dishes [11]. Moreover, fresh or shade-dried flowers of G. tridentata are also used in folk medicine, in infusions, decoctions, and tonics [12] as anti-inflammatory [13,14,15][13][14][15], diuretic and depurative of the liver [5,11,16,17][5][11][16][17]. It is commonly used to ameliorate colds [5[5][18],18], in digestive disorders [5[5][18][19][20],18,19,20], intestinal [21,22][21][22] and urologic problems [5[5][11][15][16][18],11,15,16,18], and rheumatism [5,11,16][5][11][16]. Additionally, it is also used for respiratory disorders [5[5][6][13][15][18][23],6,13,15,18,23], headaches [5], to lower blood pressure [5[5][6][18],6,18], against hypercholesterolemia [5,6,18,20,22][5][6][18][20][22] and hyperglycemia [5[5][6][11][16][17][18][23][24],6,11,16,17,18,23,24], and in weight loss programs [5].
2. Phytochemical Characterization
The main compounds found are flavonoids, as well as hydroxycinnamic acids and hydroxybenzoic acids (Table 1, Figure 21). Additionally, extracts collected in the flowering period (May), as well as flowers, presented a more diverse phytochemical profile than extracts collected during the rest of the year.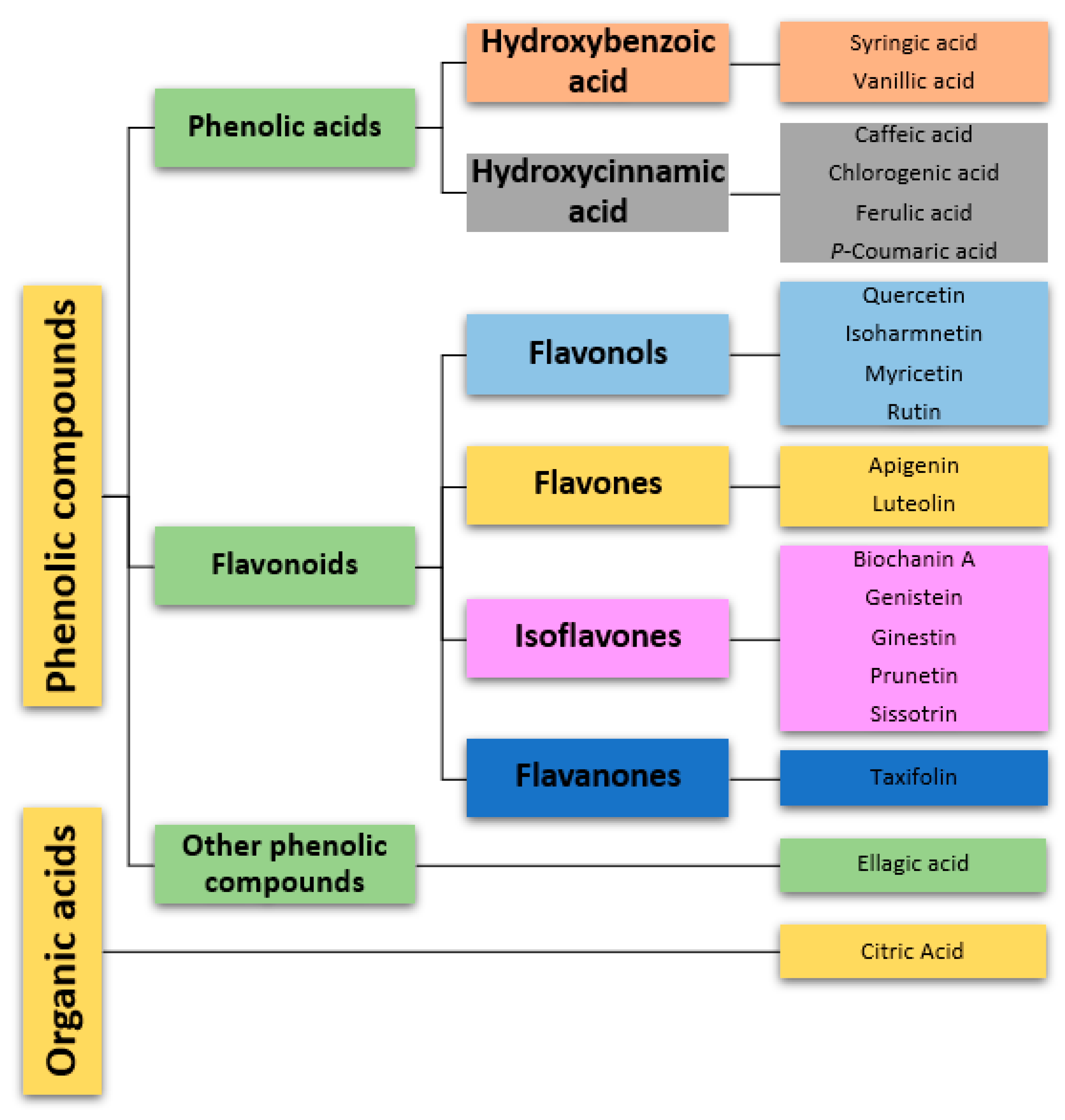
Figure 21.
Classification of phytochemical compounds identified in
Genista tridentata
. Each distinct category is associated with a unique color.
Table 1.
Major biologically active compounds were found in several samples of
G. tridentata
(X—detected; NA—not available; ND—not detected).
Table 2. Composition (%) of the essential oils of G. tridentata isolated by hydrodistillation, collected in different years and locations. (AMF02: Flowers, collected in Arneiro das Milhariças in 2002; AMF03: collected in Arneiro das Milhariças in 2003; AML02: collected in Arneiro das Milhariças in 2002; AML03: collected in Arneiro das Milhariças in 2003; PAPN: collected in Pedra de Altar, Proença a nova; PSFPN: collected in Póvoa, Sobreira Formosa, Proença a nova; SCB: collected in Sarzeda, Castelo Branco; MCSB: collected in Milhasa do Corvo, Sarzeda, Castelo Branco; ND—not detected).
| Components | Flowers | Leaves + Stems | Aerial Parts | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | [11] | [29] | [11] | |||||||||||||||||||||||||||||||
| RI | AMF02 | AMF03 | Herbal Shop | AML02 | AML03 | PAPN | PFSPNa | PFSPNb | SCB | MCSB | ||||||||||||||||||||||||
| trans-2-Hexenal | 866 | 1.6 | 0.5 | 0.1 | ND | 1.6 | ND | ND | 1.7 | 3.2 | ND | |||||||||||||||||||||||
| Part plant used | Crude | flowers | Stems and leaves | Flowers | Aerial parts | In vitro culture | ||||||||||||||||||||||||||||
| cis | Leaves + Flowers | -3-Hexen-1-ol | 868 | NA | Flowers | Flowers | ||||||||||||||||||||||||||||
| 1.6 | 1.2 | 5.3 | ND | ND | 0.8 | 3 | ND | Sampling localization | Vila Real | Serra da Estrela | Serra da Estrela | Cinfães | Montesinho | Herbal Shop—DIÉTICA ® | Malcata | Gardunha | Cinfães | Herbal Shop—Ervital | Viseu | Malcata | Gardunha | Malcata | Gardunha | Herbal shop—Ervital | ||||||||||
| cis-2-Hexen-1-ol | 882 | Herbal Shop—DIÉTICA | 1.5 | 1.2 | ND | ND | 0.8 | ND | ® | Montesinho | Viseu | Herbal shop—Ervital | ||||||||||||||||||||||
| ND | 0.6 | 1.2 | ND | Sampling period | NA | Spring | NA | May | May | NA | Spring 2012 | |||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| n | NA | February | May | February | May | NA | -Hexanol | Spring 2019 | NA | Spring 2012 | ||||||||||||||||||||||||
| 882 | 0.5 | 1.6 | ND | ND | 1.1 | ND | ND | 2019 | ||||||||||||||||||||||||||
| 1.1 | 0.7 | ND | 5,5′-Dihydroxy-3′-methoxy-isoflavone-7-O-β-glucoside | ND | ND | ND | X | ND | ND | X | X | ND | X | ND | X | X | X | X | X | X | ND | ND | ND | ND | X | |||||||||
| 5,5′-Dihydroxi-3′-methoxyisoflavone | ND | ND | ND | ND | X | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ||||||||||||
| 7-Methylorobol | ND | ND | ND | ND | ND | ND | X | ND | X | X | ND | |||||||||||||||||||||||
| ND | ND | ND | 0.3 | ND | ||||||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| Benzene acetaldehyde | 1002 | 1.8 | 1.8 | ND | 0.3 | 1.2 | ND | ND | ||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| n-Heptanal | 897 | 11.8 | 4.8 | |||||||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| ND | ||||||||||||||||||||||||||||||||||
| ND | ND | ND | ND | |||||||||||||||||||||||||||||||
| ND | ND | 0.9 | ND | 0.5 | 0.8 | ND | ND | 0.3 | ND | |||||||||||||||||||||||||
| n-Nonane | 900 | ND | ND | ND | ND | 0.2 | ND | ND | 2.3 | 0.2 | ND | |||||||||||||||||||||||
| Benzaldehyde | 927 | 0.5 | 0.8 | 0.3 | ND | 0.6 | 1 | ND | 0.6 | 0.1 | ND | ND | ND | X | X | X | ND | ND | ND | ND | ND | X | ||||||||||||
| α-Pinene | 930 | ND | 0.3 | 0.3 | ND | 0.8 | ND | ND | 0.5 | 0.1 | ND | Apigenin 5,7-dimethyl | ||||||||||||||||||||||
| n | ND | -Heptanol | 952ND | ND | ND | ND | 0.5X | 1.6 | ND | ND | 1.5ND | ND | ND | ND | ND | ND | ND | ND | ND | NDND | ND | ND | ND | ND | ND | ND | ND | ND | ||||||
| ND | 1.3 | Apigenin 5,7-dimethyl ether 4′galactoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| 1-Octen-3-ol | 961 | 10.7 | 21 | 9.2 | 11.5 | 22.6 | ND | ND | ND | ND | ND | ND | ND | 1.7 | 29.7 | 15 | ND | ND | X | ND | ND | ND | ||||||||||||
| 25.8 | 36.8 | Biochanin A | X | ND | ND | ND | ND | X | ND | ND | ||||||||||||||||||||||||
| 2-Pentyl furan | ND | X | ND | ND | ND | ND | ND | ND | ND | 972 | ND | X | ND | ND | 2.4 | X | ||||||||||||||||||
| 1.3 | 0.8 | 2.5 | 0.5 | ND | ND | 0.7 | 2.1 | 1.4 | Biochanin A O-acetylhexoside-O-hexoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | |||
| n-octanal | 973 | ND | ND | 0.6 | ND | ND | ND | ND | ND | ND | ND | Biochanin A O-hexoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 3-Octanol | X | |||||||||||||||||||||||||||||||||
| 974 | 1.4 | 1.5 | ND | 1.9 | ND | ND | ND | 1.9 | 0.3 | 1.5 | Biochanin A O-hexoside-O-hexoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | |
| Benzyl alcohol | 996 | ND | ND | ND | 0.3 | 0.4 | Biochanin A-glucoside | X | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | |||||
| 0.4 | 1.4 | 0.6 | Caffeic acid | ND | X | X | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||
| ρ | ND | -Cymene | 1003 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.3 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||||||||||||
| ND | ND | Chlorogenic acid | ND | ND | X | |||||||||||||||||||||||||||||
| 1,8-Cineole | 1005 | 0.9 | ND | ND | 1 | 0.7 | 1.1ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||||||||||
| 0.2 | ND | ND | ND | ND | ND | Citric acid | ND | ND | ND | ND | ND | ND | X | X | ||||||||||||||||||||
| Limonene | ND | ND | ND | ND | 1009 | X | 0.9 | 1 | ND | 1.1 | X | X | X | 0.2 | ND | X | ND | ND | 0.3 | NDND | ND | ND | ND | |||||||||||
| ND | Dihydroquercetin 6-C-hesoxide | ND | ND | ND | ND | X | ||||||||||||||||||||||||||||
| Acetophenone | 1017 | ND | ND | ND | ND | X | ND | ND | ND | 1.4 | ND | ND | ND | ND | 2.1 | 0.5 | ND | ND | ND | ND | NDND | ND | ND | ND | X | ND | X | |||||||
| Ellagic acid | ND | X | X | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||
| n | X | -Octanol | ND | ND | ||||||||||||||||||||||||||||||
| 1045 | 0.5 | 0.4 | 0.7 | 2.1 | 0.3 | 0.6 | ND | ND | ND | ND | Ferulic acid | ND | X | X | ND | ND | ND | ND | ND | ND | ||||||||||||||
| ρ | ND | -Cymenene | 1050 | ND | ND | ND | 0.6 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||||||||||||
| ND | ND | ND | Genistein-8-C-glucoside | ND | ND | ND | ND | ND | X | |||||||||||||||||||||||||
| Heptanoic acid | 1056 | 0.5 | 1.2 | X | X | ND | ND | ND | X | ND | 0.4 | ND | NDX | X | X | X | NDX | X | ND | X | ND | ND | X | |||||||||||
| ND | 2.1 | Ginestein | X | ND | ND | ND | ND | ND | X | X | X | X | X | X | ND | ND | X | X | ND | |||||||||||||||
| Phenyl ethyl alcohol | ND | ND | ND | X | 1064 | X | ||||||||||||||||||||||||||||
| 0.7 | 1.2 | ND | 2 | 1.7 | ND | 3.6 | 3.3 | 3.4 | 6.3 | Ginestein derivatives | X | ND | ND | ND | X | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | X | X | ND | ||
| n-Nonanal | 1073 | 14.5 | 6.1 | 6.5 | 4.6 | 0.9 | 10.5 | 4.1 | 0.2 | 0.9 | 1 | Ginestin | X | ND | ND | X | ND | ND | ND | ND | X | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X |
| Linalol | 1074 | 2.9 | 0.5 | 7.1 | ND | 2 | ND | 5.2 | ND | 2.3 | 1 | Isoquercitrin | ND | ND | ND | |||||||||||||||||||
| cis-Rose oxide | 1083 | 2.9 | X | ND | X | X | 0.5 | X | ND | ND | ND | 2X | X | X | ND | X | ND | X | X | X | NDND | X | 5.2 | 2.3 | 1ND | X | X | |||||||
| Isorhamnetin-O-hexoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||||||||||||||||||||||
| Camphor | 1095 | ND | ND | 0.7 | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ||||||||||||||||||
| ND | ND | ND | ND | ND | Luteolin-O-(O-acetyl)-glucuronide | ND | ||||||||||||||||||||||||||||
| n | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | |||||||||||||
| -Undecane | 1100 | ND | ND | ND | ND | ND | ND | 1 | 2.3 | 0.2 | ND | Luteolin-O-glucuronide | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | |
| trans | ND | |||||||||||||||||||||||||||||||||
| -Rose oxide | 1100 | ND | ND | ND | 2.1 | 0.7 | ND | 1 | ND | ND | ND | Methylbiochanin A/methylprunetin | ND | ND | ND | ND | ND | ND | ||||||||||||||||
| trans | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ||||||||||||||||||
| -Pinocarveol | 1106 | ND | ND | 0.3 | ND | ND | ND | ND | ND | 0.2 | ND | Methylbiochanin A/methylprunetin derivative | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ||||||
| 2- | ND | trans,6 cis | ND | ND | ND | ND | X | |||||||||||||||||||||||||||
| -Nonadienal | 1106 | 2.1 | 0.3 | 0.2 | ND | ND | ND | ND | ND | ND | ND | Methylbiochanin A/methylprunetin O-hexoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 2- trans | ND | -Nonen-1-al | ND | X | ||||||||||||||||||||||||||||||
| 1114 | 0.5 | 0.4 | ND | 2.2 | 0.2 | ND | ND | ND | ND | ND | Myricetin-6-C-glucoside | ND | ND | ND | ND | X | X | X | X | X | X | ND | X | X | X | X | X | X | ND | X | X | - | X | |
| Pentyl benzene | 1119 | 1.5 | ND | ND | ND | 0.3 | ND | ND | ND | ND | ND | p-Coumaric acid | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Menthone | 1120 | ND | ND | 0.2 | ND | ND | ND | ND | ND | ND | ND | Pentahydroxy-flavonol-di-O-glucoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND | ND |
| Benzyl acetate | 1123 | ND | ND | 0.2 | ND | ND | ND | ND | ND | ND | ND | Prunetin | ND | ND | ND | X | ND | ND | X | ND | X | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | X |
| Borneol | 1134 | ND | ND | 1.1 | ND | ND | ND | ND | ND | ND | ND | Quercetin | ND | X | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Lavandulol | ND | |||||||||||||||||||||||||||||||||
| 1142 | ND | ND | 0.3 | ND | ND | ND | ND | ND | ND | ND | Quercetin 3-O-galactoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | |
| Menthol | 1148 | ND | ND | 0.5 | ND | ND | ND | ND | ND | ND | ND | Quercetin deoxyhexosyl-hexoside | ND | ND | ND | ND | X | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | X |
| Terpinen-4-ol | 1148 | ND | ND | 0.7 | ND | ND | ND | ND | ND | ND | ND | Quercetin O-hexoside | ND | ND | ND | ND | X | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | X |
| Octanoic acid | 1156 | 0.3 | ND | 0.5 | 0.5 | ND | ND | ND | ND | ND | ND | Quercetin-3-O-rutinoside | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | X |
| α-Terpineol | 1159 | ND | ND | 1.8 | ND | ND | ND | 1.2 | 0.8 | 0.3 | ND | Quercetin derivates | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | X | ND | ND | ND | ND | ND | ND | ND | ND | X | X | |
| Safranal | ND | |||||||||||||||||||||||||||||||||
| 1160 | 1.4 | 0.3 | ND | ND | 0.5 | ND | ND | ND | ND | ND | Quinic acid | ND | ND | ND | ND | ND | ND | X | X | ND | ND | ND | X | X | X | X | X | X | ND | ND | ND | ND | ND | |
| Methyl chavicol (=estragole) | 1163 | ND | ND | 0.9 | ND | ND | ND | ND | ND | ND | ND | Rosmarinic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | |||
| n-Decanal | 1180 | ND | 0.3 | 0.4 | ND | ND | ND | ND | ||||||||||||||||||||||||||
| ND | ND | ND | ND | ND | ND | Rutin | ND | ND | ND | ND | ND | ND | X | X | X | ND | ND | X | X | X | X | X | X | |||||||||||
| Pulegone | ND | ND | ND | ND | 1210 | ND | ||||||||||||||||||||||||||||
| ND | ND | 1.4 | ND | ND | ND | ND | ND | ND | ND | Sissotrin | ND | ND | ND | X | ND | ND | X | X | X | X | ND | ND | ||||||||||||
| Geraniol | 1236 | 0.3 | 1.6 | 0.6 | 4 | 9.2 | 3.2 | 1 | ND | X | X | ND | ND | ND | ND | - | 1.4 | 2.8 | ND | ND | X | |||||||||||||
| Syringic acid | ND | X | X | ND | ND | |||||||||||||||||||||||||||||
| Linalyl acetate | 1245 | ND | ND | 1.4 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| Trans-Anethole | 1254 | ND | ND | 4.7 | ND | ND | ND | ND | ND | ND | ND | Taxifolin-6-C-glucoside | ND | ND | ND | ND | ND | X | X | X | ND | ND | ND | X | X | X | X | ND | ND | ND | X | ND | ||
| n | ND | -Decanol | 1259 | ND | ||||||||||||||||||||||||||||||
| 0.3 | 1.6 | 0.6 | 4 | 0.2 | 3.2 | 3.4 | 2.5 | 3.2 | 1.9 | Vanillic acid | ND | X | X | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||||||||||
| 2-Undecanone | 1273 | ND | ND | 2.2 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| Perilla alcohol | 1274 | ND | ND | ND | ND | 3.4 | ND | ND | ND | 0.6 | ND | |||||||||||||||||||||||
| Nonanoic acid | 1274 | ND | 0.3 | 1.5 | 2.3 | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| cis-Theaspirane | 1279 | 1.6 | 2.2 | ND | 12.7 | 7.1 | 14.2 | 5.3 | 13.2 | 9 | 6.2 | |||||||||||||||||||||||
| 2 trans,4 trans-Decadienal | 1285 | 0.8 | 1.3 | ND | ND | 0.1 | ND | 1.8 | ND | 2 | ND | |||||||||||||||||||||||
| cis-Transpirane | 1286 | ND | ND | 3.2 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| Carvacrol | 1286 | ND | ND | 0.3 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| 2-trans-4-trans-Decadienal | 1286 | ND | ND | 1 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| trans-Theaspirane | 1300 | 2.4 | 1.9 | 3.9 | 12.1 | 6.8 | 17.2 | 6.3 | 13.6 | 10 | 5.5 | |||||||||||||||||||||||
| Hexyl tiglate ester | 1316 | ND | ND | 0.2 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| Eugenol | 1327 | 1.4 | 1.7 | 0.8 | 3.5 | 2.6 | ND | 3.1 | 3 | 3.2 | 3.6 | |||||||||||||||||||||||
| α-Terpenyl acetate | 1334 | ND | ND | 0.3 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| α-Longipinene | 1338 | ND | ND | 0.1 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| Decanoic acid | 1350 | ND | ND | 0.8 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| trans-β-Dasmascenone | 1356 | ND | ND | 0.8 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| Geranyl acetate | 1370 | ND | ND | 0.5 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| α-Copaene | 1375 | ND | ND | ND | ND | ND | ND | 0.9 | ND | ND | ND | |||||||||||||||||||||||
| β-Bourbonene | 1379 | ND | ND | ND | ND | ND | ND | 1.5 | ND | 1.1 | ND | |||||||||||||||||||||||
| 2-Pentadecanone | 1390 | ND | ND | 0.8 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| Longifolene | 1399 | ND | ND | ND | ND | ND | ND | 1.4 | ND | ND | ND | |||||||||||||||||||||||
| β-Caryophyllene | 1414 | ND | 0.4 | 1.2 | ND | ND | ND | 2.7 | ND | 2 | 0.9 | |||||||||||||||||||||||
| Geranyl acetonea | 1434 | ND | 3.6 | 0.7 | ND | ND | ND | 1.2 | ND | 0.6 | ND | |||||||||||||||||||||||
| allo-Aromadendrene | 1456 | ND | ND | 0.7 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| trans-β-Ionone | 1456 | ND | ND | 1.1 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| Germacrene-D | 1474 | ND | 0.2 | ND | ND | ND | 9.7 | 3.3 | ND | 0.7 | ND | |||||||||||||||||||||||
| α-Curcumene | 1475 | ND | ND | 0.5 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| ƴ-Cadinene | 1500 | ND | 3.3 | ND | ND | ND | ND | 1.2 | ND | 1.1 | 1.9 | |||||||||||||||||||||||
| σ-Cadinene | 1505 | ND | 2.4 | ND | ND | ND | ND | 1.6 | ND | 2 | 1.9 | |||||||||||||||||||||||
| Dodecanoic acid | 1551 | 3.5 | 2.1 | 5.3 | 2.6 | 0.3 | 15 | ND | ND | 0.9 | 1.1 | |||||||||||||||||||||||
| β-Caryophyllene oxide | 1561 | ND | ND | ND | ND | ND | ND | 1.3 | ND | 1.2 | 2.9 | |||||||||||||||||||||||
| n-Tetradecanal | 1596 | ND | ND | ND | ND | ND | ND | 1.1 | ND | 2.7 | 1.5 | |||||||||||||||||||||||
| n-Pentadecanal; | 1688 | ND | ND | ND | ND | ND | ND | ND | ND | 0.8 | ND | |||||||||||||||||||||||
| Tetradecanoic acid | 1734 | ND | ND | 0.2 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| Hexadecanoic acid | 1779 | ND | ND | 0.7 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| 9,12-Octadecadienoic acid | 1820 | ND | ND | 0.4 | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||||||||
| % of identified components | 71.8 | 75.1 | 71.8 | 78.4 | 76.8 | 77.1 | 82.9 | 64.8 | 88.5 | 82.2 | ||||||||||||||||||||||||
| Grouped components | ||||||||||||||||||||||||||||||||||
| Monoterpene hydrocarbons | 0.9 | 1.3 | 0.6 | 1.1 | 1 | ND | ND | 0.8 | 0.1 | ND | ||||||||||||||||||||||||
| Oxygen-containing monoterpenes | 6.2 | 7 | 18.6 | 10.6 | 17.5 | 3.2 | 9.6 | 0.8 | 5.4 | 3.8 | ||||||||||||||||||||||||
| Sesquiterpene hydrocarbons | ND | 6.3 | 2.5 | ND | ND | 9.7 | 12.6 | ND | 6.9 | 4.7 | ||||||||||||||||||||||||
| Oxygen-containing sesquiterpenes | ND | ND | 7.1 | ND | ND | ND | 1.3 | ND | 1.2 | 2.9 | ||||||||||||||||||||||||
| Phenylpropanoids | 1.4 | 1.7 | 6.4 | 3.5 | 2.6 | ND | 3.1 | 3 | 3.2 | 3.6 | ||||||||||||||||||||||||
| Oil yield (v/w) | <0.05% | <0.05% | 0.01% | <0.05% | <0.05% | <0.05% | <0.05% | <0.05% | <0.05% | <0.05% | ||||||||||||||||||||||||
References
- Pinela, J.; Carvalho, A.M.; Ferreira, I.C. Wild edible plants: Nutritional and toxicological characteristics, retrieval strategies and importance for today’s society. Food Chem. Toxicol. 2017, 110, 165–188.
- Demasi, S.; Caser, M.; Donno, D.; Enri, S.R.; Lonati, M.; Scariot, V. Exploring wild edible flowers as a source of bioactive compounds: New perspectives in horticulture. Folia Hortic. 2021, 33, 27–48.
- Pinto, D.C.; Simões, M.A.; Silva, A.M. Genista tridentata L.: A rich source of flavonoids with anti-inflammatory activity. Medicines 2020, 7, 31.
- Simões, M.A.; Pinto, D.C.; Neves, B.M.; Silva, A.M. Flavonoid profile of the Genista tridentata L., a species used traditionally to treat inflammatory processes. Molecules 2020, 25, 812.
- Coelho, M.T.; Gonçalves, J.C.; Alves, V.; Moldão-Martins, M. Antioxidant activity and phenolic content of extracts from different Pterospartum tridentatum populations growing in Portugal. Procedia Food Sci. 2011, 1, 1454–1458.
- Ferreira, F.M.; Dinis, L.T.; Azedo, P.; Galhano, C.I.; Simões, A.; Cardoso, S.M.; Rosário, M.; Domingues, M.; Pereira, O.R.; Palmeira, C.M.; et al. Antioxidant capacity and toxicological evaluation of Pterospartum tridentatum flower extracts. CyTA-J. Food 2012, 10, 92–102.
- GBIF—Global Biodiversity Information Facility. Available online: https://www.gbif.org/search?q=Pterospartum%20tridentatum (accessed on 10 October 2023).
- Plants of the World Online (POWO). Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:496422-1#synonyms (accessed on 10 October 2023).
- Vitor, R.F.; Mota-Filipe, H.; Teixeira, G.; Borges, C.; Rodrigues, A.I.; Teixeira, A.; Paulo, A. Flavonoids of an extract of Pterospartum tridentatum showing endothelial protection against oxidative injury. J. Ethnopharmacol. 2004, 93, 363–370.
- Novais, M.H.; Santos, I.; Mendes, S.; Pinto-Gomes, C. Studies on pharmaceutical ethnobotany in Arrábida natural park (Portugal). J. Ethnopharmacol. 2004, 93, 183–195.
- Grosso, A.C.; Costa, M.M.; Ganço, L.; Pereira, A.L.; Teixeira, G.; Lavado, J.M.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Essential oil composition of Pterospartum tridentatum grown in Portugal. Food Chem. 2007, 102, 1083–1088.
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Influence of the drying method in the antioxidant potential and chemical composition of four shrubby flowering plants from the tribe Genisteae (Fabaceae). Food Chem. Toxicol. 2011, 49, 2983–2989.
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647.
- Bremner, P.; Rivera, D.; Calzado, M.A.; Obón, C.; Inocencio, C.; Beckwith, C.; Fiebich, B.L.; Munoz, E.; Heinrich, M. Assessing medicinal plants from south-eastern Spain for potential anti-inflammatory effects targeting nuclear factor-Kappa B and other pro-inflammatory mediators. J. Ethnopharmacol. 2009, 124, 295–305.
- Garcia-Oliveira, P.; Carreira-Casais, A.; Pereira, E.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Stojkovic, D.; Sokovic, M.; Simal-Gandara, J.; Prieto, M.A.; et al. From tradition to health: Chemical and bioactive characterization of five traditional plants. Molecules 2022, 27, 6495.
- Gonçalves, S.; Gomes, D.; Costa, P.; Romano, A. The phenolic content and antioxidant activity of infusions from Mediterranean medicinal plants. Indust. Crops Prod. 2013, 43, 465–471.
- Aires, A.; Marrinhas, E.; Carvalho, R.; Dias, C.; Saavedra, M.J. Phytochemical composition and antibacterial activity of hydroalcoholic extracts of Pterospartum tridentatum and Mentha pulegium against Staphylococcus aureus isolates. BioMed Res. Int. 2016, 2016, 5201879.
- Balanč, B.; Kalušević, A.; Drvenica, I.; Coelho, M.T.; Djordjević, V.; Alves, V.D.; Sousa, I.; Moldão-Martins, M.; Rakic, V.; Nedovic, V.; et al. Calcium–alginate–inulin microbeads as carriers for aqueous carqueja extract. J. Food Sci. 2016, 81, E65–E75.
- Gião, M.S.; González-Sanjosé, M.L.; Muñiz, P.; Rivero-Pérez, M.D.; Kosinska, M.; Pintado, M.E.; Malcata, F.X. Protection of deoxyribose and DNA from degradation by using aqueous extracts of several wild plants. J. Sci. Food Agric. 2008, 88, 633–640.
- Serralheiro, M.L.M.; Falé, P.L.; Ferreira, C.; Rodrigues, A.M.; Cleto, P.; Madeira, P.J.A.; Florêncio, M.H.; Frazão, F.N.; Serralheiro, M.L.M. Antioxidant and anti-acetylcholinesterase activity of commercially available medicinal infusions after in vitro gastrointestinal digestion. J. Med. Plants Res. 2013, 7, 1370–1378.
- Coelho, C.M.M.; de Mattos Bellato, C.; Santos, J.C.P.; Ortega, E.M.M.; Tsai, S.M. Effect of phytate and storage conditions on the development of the ‘hard-to-cook’ phenomenon in common beans. J. Sci. Food Agric. 2007, 87, 1237–1243.
- Falé, P.L.; Ferreira, C.; Rodrigues, A.; Frazão, F.; Serralheiro, M. Studies on the molecular mechanism of cholesterol reduction by Fraxinus angustifolia, Peumus boldus, Cynara cardunculus and Pterospartum tridentatum infusions. J. Med. Plants Res. 2014, 8, 9–17.
- Luis, A.; Domingues, F.; Duarte, A.P. Bioactive compounds, RP-HPLC analysis of phenolics, and antioxidant activity of some portuguese shrub species extracts. Nat. Product Commun. 2011, 6, 1863–1872.
- Gonçalves, J.C.; Coelho, M.T.; da Graça Diogo, M.; Alves, V.D.; Bronze, M.R.; Coimbra, M.A.; Martins, V.M.; Moldão-Martins, M. In vitro shoot cultures of Pterospartum tridentatum as an alternative to wild plants as a source of bioactive compounds. Nat. Product Commun. 2018, 13, 439–442.
- Paulo, A.; Martins, S.; Branco, P.; Dias, T.; Borges, C.; Rodrigues, A.I.; Costa, M.C.; Teixeira, A.; Mota-Filipe, H. The opposing effects of the flavonoids isoquercitrin and sissotrin, isolated from Pterospartum tridentatum, on oral glucose tolerance in rats. Phytother. Res. 2008, 22, 539–543.
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Scientific validation of synergistic antioxidant effects in commercialised mixtures of Cymbopogon citratus and Pterospartum tridentatum or Gomphrena globosa for infusions preparation. Food Chem. 2015, 185, 16–24.
- Gonçalves, A.C.; Bento, C.; Nunes, A.R.; Simões, M.; Alves, G.; Silva, L.R. Multitarget protection of Pterospartum tridentatum phenolic-rich extracts against a wide range of free radical species, antidiabetic activity and effects on human colon carcinoma (Caco-2) cells. J. Food Sci. 2020, 85, 4377–4388.
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Pterospartum tridentatum, Gomphrena globosa and Cymbopogon citratus: A phytochemical study focused on antioxidant compounds. Food Res. Int. 2014, 62, 684–693.
- Barbosa, P.; Lima, A.S.; Vieira, P.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Nematicidal activity of essential oils and volatiles derived from portuguese aromatic flora against the pinewood nematode. J. Nematol. 2010, 42, 8–16.
- Faria, J.M.; Barbosa, P.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from essential oils, essential oils fractions and decoction waters. Phytochemistry 2013, 94, 220–228.
- Faria, J.M.S.; Sena, I.; Ribeiro, B.; Rodrigues, A.M.; Maleita, C.M.N.; Abrantes, I.; Bennet, R.; Mota, M.; Figueiredo, A.C.D.S. First report on Meloidogyne chitwoodi hatching inhibition activity of essential oils and essential oils fractions. J. Pest Sci. 2016, 89, 207–217.
More
