Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Carlos Escobar and Version 2 by Fanny Huang.
Heart failure (HF) is associated with a high morbidity and mortality burden. In light of more recent evidence, SGLT2 inhibitors are currently recommended as first-line therapy in managing patients with HF, regardless of ejection fraction, to reduce HF burden. The DAPA-HF and DELIVER trials, and particularly, the pooled analysis of both studies, have shown that dapagliflozin significantly reduces the risk of cardiovascular death, all-cause death, total HF hospitalizations, and MACE in the whole spectrum of HF, with sustained benefits over time.
- dapagliflozin
- heart failure
1. Introduction
Heart failure (HF) is a very common syndrome worldwide. In the general adult population, the current prevalence of HF is around 2% but increases with age, reaching 7% in those patients >45 years and 16% in those over 75 years [1][2][3][1,2,3]. Of note, due to the aging of the population and the better management of acute cardiovascular conditions, it is very likely that these numbers will increase in the following years [4].
Despite traditional treatments with renin-angiotensin system inhibitors and beta blockers, death rates of patients with HF have remained unacceptably high (i.e., 40% after a median follow-up of 2.5 years) [5]. Fortunately, these numbers are improving, particularly in those countries with a higher implementation of guideline-directed medical therapy [6][7][6,7]. Along this same line, a meta-analysis of three studies, EMPHASIS-HF trial (Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure), PARADIGM-HF trial (Prospective Comparison of ARNI [Angiotensin Receptor–Neprilysin Inhibitor] with ACEI [Angiotensin-Converting–Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial) and DAPA-HF trial (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) showed that compared with the combination of an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin receptor blockers (ARB) and a beta blocker, the combination of an angiotensin receptor-neprilysin inhibitor (ARNI), a beta blocker, eplerenone (a mineralocorticoid receptor antagonist) and dapagliflozin (a sodium-glucose cotransporter 2 inhibitor [SGLT2i]) was associated with a significant 50% risk reduction of cardiovascular death and a significant 47% risk reduction of all-cause mortality [8].
Acute HF represents the first cause of hospitalization in patients over 65 years in developed countries [3]. HF hospitalizations are an inflection point in the evolution and progression of the disease. Thus, in-hospital mortality varies between 4% and 10% and mortality within the first year after discharge reaches 25–30% of cases [9][10][9,10]. In addition, the risk of rehospitalization is high, particularly during the vulnerable period that accounts for the first months after discharge [11]. The meta-analysis of the EMPHASIS-HF, PARADIGM-HF and DAPA-HF trials showed that compared with the combination of an ACEi/ARB and a beta blocker, the quadruple therapy with ARNI, beta blocker, a mineralocorticoid receptor antagonist and dapagliflozin significantly reduced the risk of cardiovascular death or HF hospital admission by 62% and also hospital admission for HF alone by 68% [8]. Furthermore, HF has an important impact on the health system’s economic burden, making HF hospitalizations the main determinant for total costs. As a result, reducing HF hospitalization may translate into a reduction in health care costs [12][13][12,13].
There are different phenotypes of HF according to ejection fraction. Thus, around 40–50% of patients may have HF with reduced ejection fraction (HFrEF), 40% HF with preserved ejection fraction (HFpEF), and around 10% HF with mildly reduced ejection fraction (HFmrEF) [1][14][15][1,14,15]. Despite the substantial differences in the clinical profile, comorbidities, and cause of death according to HF phenotypes, the risk of adverse clinical outcomes, both cardiovascular death and HF (re-)hospitalization are quite similar along the whole spectrum of left ventricular ejection fraction [1][14][15][1,14,15].
The pathogenesis of HF is complex, as different neurohormonal systems are involved, including the overactivation of deleterious pathways, such as the renin-angiotensin and the sympathetic nervous systems, but also the inhibition of protective pathways, such as the natriuretic peptides system and the nitric oxide-soluble guanylate cyclase-cGMP system [16][17][18][19][16,17,18,19]. SGLT2i promotes glucosuria, osmotic diuresis and natriuresis, improves volume status and cardiac metabolism, sodium-hydrogen exchange in the myocardium, reduces cardiac fibrosis, prevents and reverses adverse cardiac remodeling through the reduction of apoptosis, necrosis and autophagy and the improvement of myocardial oxygen supply/demand, reduce sympathetic stimulation, modulate the levels of leptin and adiponectin, improve control of risk factors, preserve renal function and decrease inflammation and oxidative stress [20][21][22][23][24][25][26][27][28][20,21,22,23,24,25,26,27,28].
The objectives of HF management should include reducing the risk of cardiovascular death and HF hospitalization and improving symptoms and quality of life [29][30][29,30]. In this context, current guidelines recommend as first-line therapy the quadruple therapy with renin-angiotensin system inhibitors, preferably with ARNI, beta-blockers, mineralocorticoid receptor antagonists (either spironolactone or eplerenone), and SGLT2i for patients with HFrEF. More recently, the use of diuretics for fluid retention and SGLT2i (dapagliflozin/empagliflozin) is also indicated for patients with HFmrEF and HFpEF [29][30][31][29,30,31] (Figure 1).
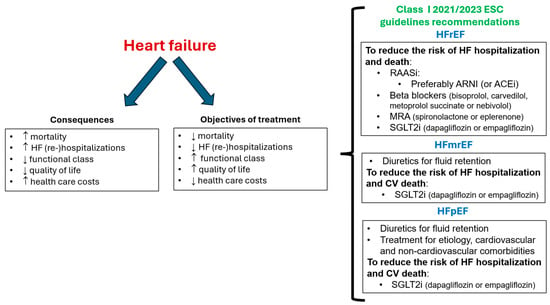
Figure 1. Objectives of treatment of heart failure and class I ESC guidelines recommendations. ARNI: angiotensin receptor-neprilysin inhibitor; CV: cardiovascular; ESC: European Society of Cardiology; HFmrEF: HF with mildly reduced ejection fraction; HFpEF: HF with preserved ejection fraction; HFrEF: HF with reduced ejection fraction; MRA: mineralocorticoid receptor antagonists; SGLT2i: sodium-glucose cotransporter 2 inhibitor; RAASi: renin-angiotensin system inhibitor.
Unfortunately, adherence to evidence from practitioners and to mediation from patients remains a problem in clinical practice, which probably translates into a higher risk of events [1][15][32][1,15,32]. Otherwise, it seems that SGLT2i may facilitate the tolerance and persistence for other evidence-based mediations [32].
2. Dapagliflozin: Beyond Heart Failure Protection
Dapagliflozin is rapidly absorbed after oral administration (Cmax of 2 h), has an absolute oral bioavailability of 78%, and can be taken with or without food. Dapagliflozin is approximately 91% protein bound. Of note, the CYP-mediated metabolism of dapagliflozin is minor, which leads to a low risk of drug-drug interactions. The mean plasma terminal half-life is around 13 h and is mainly eliminated through urinary excretion (75% in urine and 21% in feces). The dose of dapagliflozin (10 mg once daily) is not required to be adjusted according to renal or hepatic function, age, gender, race, or body weight [33][34][35][33,34,35].
The benefits of dapagliflozin have been proven not only in patients with HF but also in patients with type 2 diabetes and chronic kidney disease, providing a wide spectrum of cardiovascular protection. The DECLARE–TIMI 58 (Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58) trial included 17,160 patients with type 2 diabetes who had (40.6%) or were at risk (59.4%) for atherosclerotic cardiovascular disease. After a median follow-up of 4.2 years, compared to placebo, treatment with dapagliflozin was non-inferior with respect to MACE, a composite of cardiovascular death, myocardial infarction, or ischemic stroke (hazard ratio [HR] 0.93; 95% confidence interval [CI] 0.84–1.03), but was superior when considering those patients with prior myocardial infarction (HR 0.84; 95% CI 0.72–0.99) [36][37][36,37]. Remarkably, dapagliflozin significantly reduced the risk of cardiovascular death or hospitalization for HF (HR 0.83; 95% CI 0.73–0.95), largely due to a reduction in HF hospitalizations (HR 0.73; 95% CI 0.61–0.88) and the renal composite of ≥40% decrease in estimated glomerular filtration rate (eGFR) to <60 mL/min/1.73 m2, new end-stage renal disease, or death from renal or cardiovascular causes (HR 0.76; 95% CI 0.67–0.87) [36].
The DAPA-CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) trial included 4304 individuals with an eGFR of 25 to 75 mL/min/1.73 m2 and a urinary albumin-to-creatinine ratio of 200 to 5000 mg/g. After a median follow-up of 2.4 years (the trial was prematurely stopped because of the overwhelming efficacy of dapagliflozin), compared to placebo (on top of renin-angiotensin system inhibition), dapagliflozin significantly reduced the risk of the primary outcome composed of a sustained decline in the eGFR ≥ 50%, end-stage kidney disease, or death from renal or cardiovascular causes by 39% (HR 0.61; 95% CI 0.51–0.72), the risk of death from cardiovascular causes or HF hospitalization by 29% (HR 0.71; 95% CI 0.55–0.92) and all-cause death by 31% (HR 0.69; 95% CI 0.53–0.88) [38]. Dapagliflozin also decreased the risk of major adverse kidney and cardiovascular events and mortality for any cause regardless of the presence of type 2 diabetes [39]. The benefits of dapagliflozin were maintained even in those patients with stage 4 chronic kidney disease [40]. Moreover, dapagliflozin reduced the risk of kidney failure and cardiovascular death or HF hospitalization and improved survival in patients with chronic kidney disease, regardless of the baseline presence of prior HF [41].
3. Dapagliflozin and Heart Failure
Although the most important clinical trials that have assessed the role of dapagliflozin among the HF population are the DAPA-HF and DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trials [42][43][42,43], many other studies have been involved in the HF clinical development of dapagliflozin. For example, the DEFINE-HF (Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients with HF with Reduced Ejection Fraction) trial was an investigator-initiated, multicentre, randomized controlled trial of 263 patients with NYHA II-III HFrEF, eGFR ≥ 30 mL/min/1.73 m2, and elevated natriuretic peptides. Patients received dapagliflozin 10 mg daily or placebo for 12 weeks. Although natriuretic peptide levels were not significantly modified by dapagliflozin, there were significantly more patients experiencing clinically meaningful improvements in HF-related health status assessed by the Kansas City Cardiomyopathy Questionnaire or natriuretic peptides, regardless of type 2 diabetes status [44]. In addition, this study suggested a direct effect of dapagliflozin on more effective “decongestion”, as dapagliflozin improved lung fluid volumes measured by remote dielectric sensing after 12 weeks of therapy [45]. A pooled patient-level analysis from DEFINE-HF (263 patients with symptomatic HFrEF) and PRESERVED-HF (Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients with Preserved Ejection Fraction Heart Failure that included 324 patients with symptomatic HFpEF and elevated natriuretic peptides) trials showed that after 12 weeks of treatment, dapagliflozin improved symptoms and reduced physical limitations across the full range of ejection fraction [46]. The evidence, supporting the role of dapagliflozin on short-term maximal functional capacity was assessed in the DAPA-VO2 randomized clinical trial. This study showed that treatment with dapagliflozin led to a significant short-term improvement in peak oxygen consumption at 1 and 3 months in patients with stable HFrEF, compared to placebo [47].
