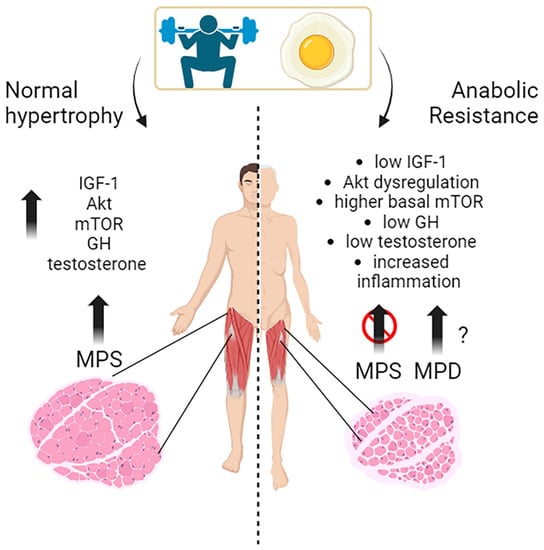
Muscle growth, or muscle hypertrophy, is a complex process regulated by several molecular pathways. The IGF-1/PI3K/Akt/mTOR pathway is a vital signaling cascade in muscle growth that involves various interconnected mechanisms. Its activation increases protein synthesis, reduces protein degradation, and improves cell growth. Akt activation is crucial in promoting muscle protein synthesis in response to exercise and nutrient intake in young individuals. The timing of exercise and protein intake also affect Akt activation and subsequent muscle protein synthesis. While exercise alone did not increase Akt and mTOR phosphorylation, protein ingestion afterward did so in a dose-dependent manner. Growth hormone (GH) promotes the uptake of essential nutrients, such as glucose and amino acids, into muscle cells for energy production and protein synthesis. Testosterone is one of the most potent naturally secreted androgenic-anabolic hormones, and its biological effects include promoting muscle growth.
- exercise
- nutrition
- skeletal muscle
1. The Molecular Mechanisms behind Muscles Growth in Young Subjects: Exercise, Nutrients, and Hormones
2. Effects of Substrates and Exercise on Skeletal Muscle Protein Synthesis in Young, Middle-Aged Subjects
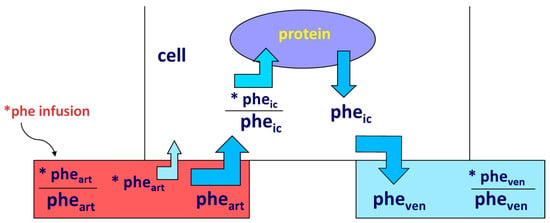
Accretion of muscle mass depends on physiological, metabolic, and hormonal factors, as well as on physical activity. Conversely, it is hindered by inactivity, malnutrition, overt diseases, and/or subtle, chronic pathological conditions [19][20][21][22][23][24]. The main protein-anabolic factors, at both the whole-body and skeletal muscle level, are the proteins and/or the amino acids themselves (i.e., the protein building blocks), as well as physical activity/exercise. In addition, energy availability, anabolic hormones (insulin, human Growth Hormone (hGH), IGF-1, β-agonists, anabolic steroids, and see also above), adequate tissue perfusion, and, in general, a “healthy status” (i.e., the absence of both overt diseases and subtle pathological conditions, such as a chronic sub-inflammatory status) also condition skeletal muscle accretion. Furthermore, the effects of any of these factors could be divided into either “acute” (i.e., detectable under acute experimental conditions) or “chronic” (i.e., following repeated stimuli, with end-point results tested sometime after). Protein anabolism is thus achieved through the stimulation of protein synthesis and/or the inhibition of protein degradation. MPS can be determined in vivo either by measuring the incorporation of infused amino acid stable isotopes into muscle by biopsy (Figure 2) and/or through measurements of the A-V difference in labeled as well as unlabeled amino acid(s) across a sampled district predominantly constituted by skeletal muscle, typically a limb (either the leg or the forearm) [25] (Figure 3).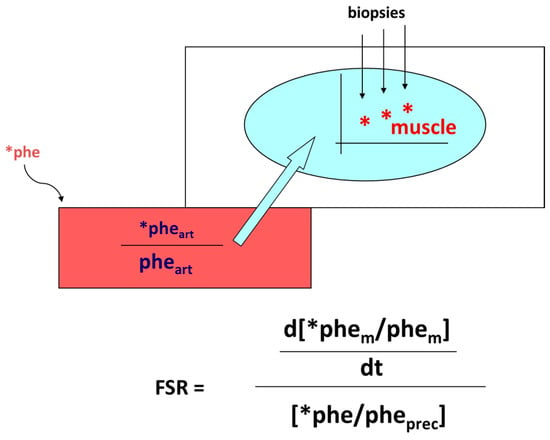
2.1. Effects of Proteins and Amino Acids
2.2. Effects of Exercise and Nutrition on Muscle Protein Synthesis and Accretion
Exercise is a potent stimulator of muscle protein synthesis, particularly in the recovery phase [49], and it positively interacts with protein/amino acid ingestion in the stimulation of skeletal muscle anabolism. Muscle mass accretion following resistance exercise combined with food ingestion is observed even following an adequate habitual protein intake (≥0.8 g kg−1 day−1) [50]. A greater protein intake (1.8–3.0 g kg−1 day−1) further augments lean body mass (i.e., protein) accretion without increasing fat mass, when compared to an energy-rich, low-protein intake (≈5% of energy as protein) [50]. The high-quality whey protein, combined with resistance exercise in young adults, exerted a greater effect on MPS than equivalent doses of lower-quality proteins, such as soy protein or casein, an effect, however, still present up to 3–5 h post-exercise [38]. As reported above, a 20 g dose of whey protein was sufficient for the maximal stimulation of post-absorptive myofibrillar MPS in young men, whether or not exercising [37], and it was effective up to 3–5 h after exercise [28][51]. Conversely, others reported that the response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g rather than 20 g of ingested whey protein [52]. After the ingestion of incremental doses of mixed milk protein (0, 15, 30, or 45 g) together with 45 g carbohydrate, the 30 g protein dose was sufficient to maximize the myosin synthesis rates during recovery from a single bout of endurance exercise in young men [53]. The intake of another high-quality protein, i.e., egg protein, as low as 5–10 g (approximating that contained in a single ≈ 60 g egg: ≈ 6.8 g total protein), increased MPS above basal following resistance exercise, reaching a maximum with a dose of 20 g egg protein [54].2.3. Effect of Other Substrates
Protein synthesis is an energy-requiring process [55]; therefore, energy-providing substrates such as glucose and fat may affect protein turnover too. The activities of the cellular pathways controlling protein turnover are bio-energetically expensive and therefore depend on intracellular energy availability (i.e., macronutrient intake) [56].Glucose
Glucose increased muscle protein synthesis in vitro [57]. Testing glucose-induced or derived substrates separately, tissue ATP decreased during incubation with lactate, and lactate + pyruvate supported protein synthesis better than pyruvate or glucose. The data on the effects in humans of either glucose or fat on protein metabolism, specifically on skeletal muscle, are scarce, complex, and not univocal [30]. Enteral glucose administration did not affect either duodenal mucosal protein FSR or the activities of mucosal proteases [58]. An oral glucose load, and the simultaneous glucose-induced stimulation of insulin secretion, did not alter the rate of whole-body protein synthesis or breakdown [59].Lipids and Ketones
The effects of either lipid or ketone infusion/administration in humans are complex. Lipid infusion in humans did not affect proteolysis [60]. In contrast, medium-chain fatty acid infusion apparently increased leucine oxidation and, therefore, net protein catabolism [61]. Thus, the effects on whole-body protein degradation may depend on the fatty acid length [62]. The increase in FFA decreased basal muscle protein synthesis, but not the anabolic effect of leucine [63]. When associated with dietary protein ingestion, neither acute nor short-term dietary fat overload impaired skeletal MPS in overweight/obese men in the post-prandial phase, thus excluding a role by dietary accumulation of intramuscular lipids on the anabolic response to meal ingestion [64]. The infusion of 3OHButyrate decreased both whole body and forearm protein turnover (measured by phenylalanine/tyrosine tracers), as well as phenylalanine catabolism, in post-absorptive conditions, whereas it did not modify the insulin-induced effects following an euglycemic clamp [60].Other Nutritional Interventions
β-alanine supplementation may increase physical performance in middle-aged individuals [65] and improve physical performance during exercise [66]. Creatine may increase muscle mass in combination with resistance exercise, although the mechanism(s) of action remain elusive. Short-term creatine monohydrate (CrM) supplementation may exert anti-catabolic actions on selected proteins in men, but it did not enhance either whole-body or mixed-muscle protein synthesis [67]. Acute metabolic studies testing various substances may provide useful information for estimating the efficacy of potentially anabolic agents [68].3. Hormones and Related Drug Interventions
3.1. Insulin
Although insulin has an undisputed anabolic effect on tissue protein, its experimental demonstration in vivo has challenged the investigators over time, mainly because its administration induces a decrease in amino acid plasma concentration, thus possibly obscuring its direct effect in muscle. A major advancement was the maintenance of the “amino acid “clamp” at baseline during insulin infusion or injection, also achieved when insulin was directly infused into the artery perfusing a muscle-rich organ (such as the leg or the forearm), thus avoiding a system perturbation of the aminoacidemia and allowing, at the same time, the insulin effect in the perfused limb to be studied selectively, using any of these techniques. Muscle protein synthesis and degradation were determined by combining amino acid isotope infusion with arterial–venous limb catheterization, often complemented by muscle biopsy in some studies.3.2. Glucocorticoids
Cortisol, often referred to as a stress hormone, has both catabolic and anabolic effects, depending on the context. Cortisol-induced secondary sarcopenia (i.e., specifically induced either by either an exogenous administration or an endocrine disease) is a frequent finding in the clinical setting. Chronic glucocorticoid exposure induces loss of lean body mass by decreasing protein synthesis and increasing degradation [69][70][71]. The protein-catabolic effect of prednisone is antagonized by growth hormone [70]. In humans, the administration of 8 mg dexamethasone daily for 4 days antagonized the anti-proteolytic effect of insulin in the forearm [72].3.3. Human Growth Hormone (hGH) and IGF-1
In humans, hGH administration increased whole-body [70] and muscle protein synthesis [73]. The insulin-like growth factor-binding proteins (IGFBPs) bind to IGFs, modulating their activity and availability. They help in regulating IGFs’ actions in various tissues, including muscle. When rhIGF-I was infused at a rate achieving plasma IGF-I concentrations close to those observed following rhGH treatment, and yet avoiding the IGF-1-induced hypoglycemia, proteolysis and protein synthesis were not affected, even in the presence of prednisone treatment [74]. However, when rhGH and rhIGF-I were administered simultaneously, nitrogen balance was remarkably improved [74]. IGF-1 exhibits several splicing variants, IGF-1Ea, which is a circulating factor synthesized in the liver, and IGF-1Eb and IGF-1Ec, recognized as Mechano-Growth Factors (MGFs), which manifest in skeletal muscles of rodents and humans, respectively [75]. The act of stretching or overloading skeletal muscles triggers a rise in IGF-1 mRNA, particularly emphasizing the specific IGF-1Ec variant (MGF) [75]. The extent to which IGF-1 alternative splicing variants might induce greater hypertrophy compared to IGF-1 per se remains partially unresolved [76]. Notably, the effects of the synthetic E-domain peptide mimetic have been elucidated: the MGF-24aa-E peptide (YQPPSTNKNTKSQRRKGSTFEEHK) activates satellite cells, prompting their replication [75].3.4. Catecholamines
Epinephrine has both an α- and β-receptor affinity. Epinephrine infusion in humans depressed plasma amino acid concentrations, particularly the essential ones, however without changing leucine or phenylalanine flux [77], nor did it impair the disposal of exogenous amino acids in humans [78]. However, in another study, the increase in plasma epinephrine concentrations inhibited proteolysis and leucine oxidation in humans via beta-adrenergic mechanism, compatible with an anabolic effect [79].3.5. Estrogens
Although estrogens are female sex hormones, they are also present in smaller amounts in males. Estrogen plays a role in maintaining bone health, promoting protein synthesis and muscle growth, and influencing body composition, in both males and females. While the exact mechanisms by which estrogens affect muscle growth are still being studied, research suggests that they can, directly and indirectly, affect muscle tissue. One way estrogens may influence muscle growth is by promoting protein synthesis, interacting with receptors in muscle cells, and activating signaling pathways involved in protein synthesis [80]. Decreased estrogen-associated signaling impairs mitochondrial function leading to muscle atrophy [81]. Estrogens can also indirectly affect muscle growth by modulating the production and activity of other hormones, such as growth hormone and insulin-like growth factor 1 (IGF-1), which are important for muscle development. Estrogens may regulate the release of these hormones from the pituitary gland and the liver, thereby influencing muscle growth [82].3.6. Androgens
Testosterone is a potent anabolic stimulus primarily through improvement in the re-utilization of amino acids released from protein degradation [83] (see also the above paragraph), and this will be further discussed below. Testosterone and progesterone, but not estradiol, stimulated muscle protein synthesis in postmenopausal women [84].
4. Exercise
Exercise is a powerful stimulus to promote skeletal muscle protein synthesis and net protein anabolism, involving specific metabolic and morphological adaptations in muscle [49], also interacting with substrates [85][86]. Exercise produces diverse changes in amino acid metabolism and protein turnover in muscle, according to the exercise phase. Acute changes in amino acid metabolism during exercise are primarily catabolic (i.e., increased amino acid oxidation), yet exercise does not cause muscle wasting. This is because both immediate and later post-exercise phases are anabolic. Thus, regular exercise is essential for optimizing muscle growth and hypertrophy. The type of exercise also determines the magnitudes of these processes, and exercise requires a sequence of metabolic adjustments from the catabolic period of the ongoing exercise to the anabolic period of recovery. Two primary exercise types commonly associated with muscle growth are resistance and endurance. Resistance exercise, such as weightlifting, involves using external resistance to challenge the muscles. This type of exercise is a primary intervention used to develop strength and stimulate muscle hypertrophy. Muscle mass increases constitute key components of conditioning in the outcome of various sports due to the correlation between cross-sectional muscle area and muscle strength [87][88].4.1. Resistance Exercise Can Be Further Classified into Two Main Categories
High-Intensity Resistance Exercise: This exercise type typically involves lifting heavy weights for a relatively low number of repetitions. It primarily targets fast-twitch muscle fibers, which have a higher potential for muscle growth. High-intensity resistance exercise promotes muscle hypertrophy by acutely causing mechanical tension and muscle damage, subsequently triggering muscle protein synthesis and adaptation [89]. High-intensity resistance exercise is a potent stimulus for skeletal MPS and hypertrophy in young adults during high-intensity exercise and post-exercise recovery [90][91][92][93][94][95]. Moderate-Intensity Resistance Exercise: This exercise type involves using moderate weights for more repetitions. While the hypertrophic response may be less pronounced than high-intensity resistance exercise, moderate-intensity resistance exercise still contributes to muscle growth and can benefit endurance and functional capacity [89].4.2. Effects of Exercise in Conjunction with Nutrient Intake
The specific adaptation to each type of exercise can vary depending on factors such as exercise intensity, volume, frequency, and individual characteristics. Combining resistance and endurance exercises in a well-designed training program can provide comprehensive benefits for muscle growth, strength, endurance, and overall fitness. It has been shown that combined strength and endurance training in the evening may lead to larger gains in muscle mass [96]. Additionally, other factors such as nutrition, rest, and recovery play crucial roles in maximizing the benefits of exercise and promoting muscle growth. Adequate protein intake, overall caloric balance, and appropriate recovery periods are important factors to be considered when optimizing muscle growth in response to exercise. During the acute phase, the energy needs to stimulate amino acid oxidation/catabolism (together with that of glucose and fat), thus depleting intracellular amino acid pools. In contrast, in the recovery phase, the depleted amino acid pools and energy need to be reconstituted. Since the response of muscle protein metabolism to a resistance exercise bout lasts up to 5 h [38], such an ample post-exercise recovery phase allows a comfortable, positive anabolic interaction with food (protein) intake. Immediately following exercise, muscle protein turnover, i.e., protein synthesis, breakdown, and amino acid transport, are accelerated. However, the net protein balance remains negative (i.e., catabolic or not above zero) unless food is ingested [85]. Therefore, food intake associated with exercise is necessary to bring muscle protein balance to be positive.4.3. Exercise–Insulin Interaction
Insulin and exercise can positively interact in the stimulation of protein anabolism, in a complex fashion. Both protein synthesis and degradation rates are ≥1-fold greater following the post-exercise recovery than at rest [97]. The effects of insulin on muscle protein synthesis and degradation, either without or with exercise, are complex. Insulin-stimulated protein synthesis at rest, but not in the recovery post-exercise phase. In contrast, insulin decreased protein degradation following exercise as opposed to no effect at rest. Thus, the post-exercise phase can be viewed as a (transient) insulin-resistant condition as regards protein synthesis that, in terms of the effects on net protein balance, is however overwhelmed by a greater insulin-mediated suppression of proteolysis.References
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970.
- Drummond, M.J.; Fry, C.S.; Glynn, E.L.; Dreyer, H.C.; Dhanani, S.; Timmerman, K.L.; Volpi, E.; Rasmussen, B.B. Rapamycin Administration in Humans Blocks the Contraction-Induced Increase in Skeletal Muscle Protein Synthesis: Rapamycin Blocks Protein Synthesis in Human Muscle. J. Physiol. 2009, 587, 1535–1546.
- Francaux, M.; Demeulder, B.; Naslain, D.; Fortin, R.; Lutz, O.; Caty, G.; Deldicque, L. Aging Reduces the Activation of the MTORC1 Pathway after Resistance Exercise and Protein Intake in Human Skeletal Muscle: Potential Role of REDD1 and Impaired Anabolic Sensitivity. Nutrients 2016, 8, 47.
- Léger, B.; Cartoni, R.; Praz, M.; Lamon, S.; Dériaz, O.; Crettenand, A.; Gobelet, C.; Rohmer, P.; Konzelmann, M.; Luthi, F.; et al. Akt Signalling through GSK-3beta, MTOR and Foxo1 Is Involved in Human Skeletal Muscle Hypertrophy and Atrophy. J. Physiol. 2006, 576, 923–933.
- Di Camillo, B.; Eduati, F.; Nair, S.K.; Avogaro, A.; Toffolo, G.M. Leucine Modulates Dynamic Phosphorylation Events in Insulin Signaling Pathway and Enhances Insulin-Dependent Glycogen Synthesis in Human Skeletal Muscle Cells. BMC Cell. Biol. 2014, 15, 9.
- Kakigi, R.; Yoshihara, T.; Ozaki, H.; Ogura, Y.; Ichinoseki-Sekine, N.; Kobayashi, H.; Naito, H. Whey Protein Intake after Resistance Exercise Activates MTOR Signaling in a Dose-Dependent Manner in Human Skeletal Muscle. Eur. J Appl. Physiol. 2014, 114, 735–742.
- Jacquemin, V.; Furling, D.; Bigot, A.; Butler-Browne, G.S.; Mouly, V. IGF-1 Induces Human Myotube Hypertrophy by Increasing Cell Recruitment. Exp. Cell Res. 2004, 299, 148–158.
- Barclay, R.D.; Burd, N.A.; Tyler, C.; Tillin, N.A.; Mackenzie, R.W. The Role of the IGF-1 Signaling Cascade in Muscle Protein Synthesis and Anabolic Resistance in Aging Skeletal Muscle. Front. Nutr. 2019, 6, 146.
- Hansen, M.; Koskinen, S.O.; Petersen, S.G.; Doessing, S.; Frystyk, J.; Flyvbjerg, A.; Westh, E.; Magnusson, S.P.; Kjaer, M.; Langberg, H. Ethinyl Oestradiol Administration in Women Suppresses Synthesis of Collagen in Tendon in Response to Exercise: Oral Contraceptives and Tendon Collagen. J. Physiol. 2008, 586, 3005–3016.
- Perrini, S.; Laviola, L.; Carreira, M.C.; Cignarelli, A.; Natalicchio, A.; Giorgino, F. The GH/IGF1 Axis and Signaling Pathways in the Muscle and Bone: Mechanisms Underlying Age-Related Skeletal Muscle Wasting and Osteoporosis. J. Endocrinol. 2010, 205, 201–210.
- Chikani, V.; Ho, K.K.Y. Action of GH on Skeletal Muscle Function: Molecular and Metabolic Mechanisms. J. Mol. Endocrinol. 2014, 52, R107–R123.
- West, D.W.D.; Phillips, S.M. Anabolic Processes in Human Skeletal Muscle: Restoring the Identities of Growth Hormone and Testosterone. Physician Sports Med. 2010, 38, 97–104.
- Florini, J.R. Effects of Testosterone on Qualitative Pattern of Protein Synthesis in Skeletal Muscle. Biochemistry 1970, 9, 909–912.
- Mauras, N.; Hayes, V.; Welch, S.; Rini, A.; Helgeson, K.; Dokler, M.; Veldhuis, J.D.; Urban, R.J. Testosterone Deficiency in Young Men: Marked Alterations in Whole Body Protein Kinetics, Strength, and Adiposity. J. Clin. Endocrinol. Metab. 1998, 83, 1886–1892.
- Demling, R.H.; Orgill, D.P. The Anticatabolic and Wound Healing Effects of the Testosterone Analog Oxandrolone after Severe Burn Injury. J. Crit. Care 2000, 15, 12–17.
- Vingren, J.L.; Kraemer, W.J.; Ratamess, N.A.; Anderson, J.M.; Volek, J.S.; Maresh, C.M. Testosterone Physiology in Resistance Exercise and Training: The Up-Stream Regulatory Elements. Sports Med. 2010, 40, 1037–1053.
- Vierck, J. Satellite Cell Regulation Following Myotrauma Caused by Resistance Exercise. Cell Biol. Int. 2000, 24, 263–272.
- Bhasin, S.; Woodhouse, L.; Casaburi, R.; Singh, A.B.; Bhasin, D.; Berman, N.; Chen, X.; Yarasheski, K.E.; Magliano, L.; Dzekov, C.; et al. Testosterone Dose-Response Relationships in Healthy Young Men. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1172–E1181.
- Plotkin, D.L.; Delcastillo, K.; Van Every, D.W.; Tipton, K.D.; Aragon, A.A.; Schoenfeld, B.J. Isolated Leucine and Branched-Chain Amino Acid Supplementation for Enhancing Muscular Strength and Hypertrophy: A Narrative Review. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 292–301.
- Tezze, C.; Amendolagine, F.I.; Nogara, L.; Baraldo, M.; Ciciliot, S.; Arcidiacono, D.; Zaramella, A.; Masiero, G.; Ferrarese, G.; Realdon, S.; et al. A Combination of Metformin and Galantamine Exhibits Synergistic Benefits in the Treatment of Sarcopenia. JCI Insight 2023, 8, e168787.
- Malena, A.; Pennuto, M.; Tezze, C.; Querin, G.; D’Ascenzo, C.; Silani, V.; Cenacchi, G.; Scaramozza, A.; Romito, S.; Morandi, L.; et al. Androgen-Dependent Impairment of Myogenesis in Spinal and Bulbar Muscular Atrophy. Acta Neuropathol. 2013, 126, 109–121.
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419.
- Tezze, C.; Romanello, V.; Desbats, M.A.; Fadini, G.P.; Albiero, M.; Favaro, G.; Ciciliot, S.; Soriano, M.E.; Morbidoni, V.; Cerqua, C.; et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab. 2017, 25, 1374–1389.
- Solagna, F.; Tezze, C.; Lindenmeyer, M.T.; Lu, S.; Wu, G.; Liu, S.; Zhao, Y.; Mitchell, R.; Meyer, C.; Omairi, S.; et al. Pro-Cachectic Factors Link Experimental and Human Chronic Kidney Disease to Skeletal Muscle Wasting Programs. J. Clin. Investig. 2021, 131, e135821.
- Smith, K.; Rennie, M.J. The Measurement of Tissue Protein Turnover. Baillière’s Clin. Endocrinol. Metab. 1996, 10, 469–495.
- Gorissen, S.H.M.; Rémond, D.; Van Loon, L.J.C. The Muscle Protein Synthetic Response to Food Ingestion. Meat Sci. 2015, 109, 96–100.
- Wolfe, R.R. Regulation of Muscle Protein by Amino Acids. J. Nutr. 2002, 132, 3219S–3224S.
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of Whey Hydrolysate, Casein, or Soy Protein Isolate: Effects on Mixed Muscle Protein Synthesis at Rest and Following Resistance Exercise in Young Men. J. Appl. Physiol. 2009, 107, 987–992.
- Bennet, W.M.; Connacher, A.A.; Scrimgeour, C.M.; Rennie, M.J. The Effect of Amino Acid Infusion on Leg Protein Turnover Assessed by L-Phenylalanine and L-Leucine Exchange. Eur. J. Clin. Investig. 2008, 20, 41–50.
- Tessari, P.; Barazzoni, R.; Zanetti, M.; Kiwanuka, E.; Tiengo, A. The Role of Substrates in the Regulation of Protein Metabolism. Baillière’s Clin. Endocrinol. Metab. 1996, 10, 511–532.
- Nygren, J.; Nair, K.S. Differential Regulation of Protein Dynamics in Splanchnic and Skeletal Muscle Beds by Insulin and Amino Acids in Healthy Human Subjects. Diabetes 2003, 52, 1377–1385.
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential Amino Acids Are Primarily Responsible for the Amino Acid Stimulation of Muscle Protein Anabolism in Healthy Elderly Adults. Am. J. Clin. Nutr. 2003, 78, 250–258.
- Kimball, S.R.; Jefferson, L.S. Regulation of Global and Specific MRNA Translation by Oral Administration of Branched-Chain Amino Acids. Biochem. Biophys. Res. Commun. 2004, 313, 423–427.
- Ham, D.J.; Caldow, M.K.; Lynch, G.S.; Koopman, R. Leucine as a Treatment for Muscle Wasting: A Critical Review. Clin. Nutr. 2014, 33, 937–945.
- Tessari, P.; Zanetti, M.; Barazzoni, R.; Vettore, M.; Michielan, F. Mechanisms of Postprandial Protein Accretion in Human Skeletal Muscle. Insight from Leucine and Phenylalanine Forearm Kinetics. J. Clin. Investig. 1996, 98, 1361–1372.
- Gwin, J.A.; Church, D.D.; Wolfe, R.R.; Ferrando, A.A.; Pasiakos, S.M. Muscle Protein Synthesis and Whole-Body Protein Turnover Responses to Ingesting Essential Amino Acids, Intact Protein, and Protein-Containing Mixed Meals with Considerations for Energy Deficit. Nutrients 2020, 12, 2457.
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar Muscle Protein Synthesis Rates Subsequent to a Meal in Response to Increasing Doses of Whey Protein at Rest and after Resistance Exercise. Am. J. Clin. Nutr. 2014, 99, 86–95.
- Moore, D.R.; Tang, J.E.; Burd, N.A.; Rerecich, T.; Tarnopolsky, M.A.; Phillips, S.M. Differential Stimulation of Myofibrillar and Sarcoplasmic Protein Synthesis with Protein Ingestion at Rest and after Resistance Exercise. J. Physiol. 2009, 587, 897–904.
- Robinson, M.J.; Burd, N.A.; Breen, L.; Rerecich, T.; Yang, Y.; Hector, A.J.; Baker, S.K.; Phillips, S.M. Dose-Dependent Responses of Myofibrillar Protein Synthesis with Beef Ingestion Are Enhanced with Resistance Exercise in Middle-Aged Men. Appl. Physiol. Nutr. Metab. 2013, 38, 120–125.
- Paddon-Jones, D.; Sheffield-Moore, M.; Zhang, X.-J.; Volpi, E.; Wolf, S.E.; Aarsland, A.; Ferrando, A.A.; Wolfe, R.R. Amino Acid Ingestion Improves Muscle Protein Synthesis in the Young and Elderly. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E321–E328.
- Fujita, S.; Volpi, R. Nutrition and sarcopenia of ageing. Nutr. Res. Rev. 2004, 17, 69–76.
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic Signaling Deficits Underlie Amino Acid Resistance of Wasting, Aging Muscle. FASEB J. 2005, 19, 422–424.
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein Intake and Exercise for Optimal Muscle Function with Aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936.
- Van Vliet, S.; Beals, J.W.; Holwerda, A.M.; Emmons, R.S.; Goessens, J.P.; Paluska, S.A.; De Lisio, M.; Van Loon, L.J.C.; Burd, N.A. Time-Dependent Regulation of Postprandial Muscle Protein Synthesis Rates after Milk Protein Ingestion in Young Men. J. Appl. Physiol. 2019, 127, 1792–1801.
- Monteyne, A.J.; Dunlop, M.V.; Machin, D.J.; Coelho, M.O.C.; Pavis, G.F.; Porter, C.; Murton, A.J.; Abdelrahman, D.R.; Dirks, M.L.; Stephens, F.B.; et al. A Mycoprotein-Based High-Protein Vegan Diet Supports Equivalent Daily Myofibrillar Protein Synthesis Rates Compared with an Isonitrogenous Omnivorous Diet in Older Adults: A Randomised Controlled Trial. Br. J. Nutr. 2021, 126, 674–684.
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and Fast Dietary Proteins Differently Modulate Postprandial Protein Accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935.
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The Digestion Rate of Protein Is an Independent Regulating Factor of Postprandial Protein Retention. Am. J. Physiol. -Endocrinol. Metab. 2001, 280, E340–E348.
- Reidy, P.T.; Walker, D.K.; Dickinson, J.M.; Gundermann, D.M.; Drummond, M.J.; Timmerman, K.L.; Cope, M.B.; Mukherjea, R.; Jennings, K.; Volpi, E.; et al. Soy-Dairy Protein Blend and Whey Protein Ingestion after Resistance Exercise Increases Amino Acid Transport and Transporter Expression in Human Skeletal Muscle. J. Appl. Physiol. 2014, 116, 1353–1364.
- Tipton, K.D.; Wolfe, R.R. Exercise, Protein Metabolism, and Muscle Growth. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 109–132.
- Churchward-Venne, T.A.; Murphy, C.H.; Longland, T.M.; Phillips, S.M. Role of Protein and Amino Acids in Promoting Lean Mass Accretion with Resistance Exercise and Attenuating Lean Mass Loss during Energy Deficit in Humans. Amino Acids 2013, 45, 231–240.
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; Van Loon, L.J. Whey Protein Stimulates Postprandial Muscle Protein Accretion More Effectively than Do Casein and Casein Hydrolysate in Older Men. Am. J. Clin. Nutr. 2011, 93, 997–1005.
- Macnaughton, L.S.; Wardle, S.L.; Witard, O.C.; McGlory, C.; Hamilton, D.L.; Jeromson, S.; Lawrence, C.E.; Wallis, G.A.; Tipton, K.D. The Response of Muscle Protein Synthesis Following Whole-Body Resistance Exercise Is Greater Following 40 g than 20 g of Ingested Whey Protein. Physiol. Rep. 2016, 4, e12893.
- Churchward-Venne, T.A.; Pinckaers, P.J.M.; Smeets, J.S.J.; Betz, M.W.; Senden, J.M.; Goessens, J.P.B.; Gijsen, A.P.; Rollo, I.; Verdijk, L.B.; van Loon, L.J.C. Dose-Response Effects of Dietary Protein on Muscle Protein Synthesis during Recovery from Endurance Exercise in Young Men: A Double-Blind Randomized Trial. Am. J. Clin. Nutr. 2020, 112, 303–317.
- Breen, L.; Phillips, S.M. Skeletal Muscle Protein Metabolism in the Elderly: Interventions to Counteract the “anabolic Resistance” of Ageing. Nutr. Metab. 2011, 8, 68.
- Reeds, P.J.; Burrin, D.G.; Davis, T.A.; Stoll, B. Amino Acid Metabolism and the Energetics of Growth. Arch. Anim. Nutr. 1998, 51, 187–197.
- Smiles, W.J.; Hawley, J.A.; Camera, D.M. Effects of Skeletal Muscle Energy Availability on Protein Turnover Responses to Exercise. J. Exp. Biol. 2016, 219, 214–225.
- Hedden, M.P.; Buse, M.G. Effects of Glucose, Pyruvate, Lactate, and Amino Acids on Muscle Protein Synthesis. Am. J. Physiol. 1982, 242, E184–E192.
- Goichon, A.; Coëffier, M.; Claeyssens, S.; Lecleire, S.; Cailleux, A.-F.; Bôle-Feysot, C.; Chan, P.; Donnadieu, N.; Lerebours, E.; Lavoinne, A.; et al. Effects of an Enteral Glucose Supply on Protein Synthesis, Proteolytic Pathways, and Proteome in Human Duodenal Mucosa. Am. J. Clin. Nutr. 2011, 94, 784–794.
- Bowtell, J.L.; Leese, G.P.; Smith, K.; Watt, P.W.; Nevill, A.; Rooyackers, O.; Wagenmakers, A.J.; Rennie, M.J. Effect of Oral Glucose on Leucine Turnover in Human Subjects at Rest and during Exercise at Two Levels of Dietary Protein. J. Physiol. 2000, 525 Pt 1, 271–281.
- Thomsen, H.H.; Rittig, N.; Johannsen, M.; Møller, A.B.; Jørgensen, J.O.; Jessen, N.; Møller, N. Effects of 3-Hydroxybutyrate and Free Fatty Acids on Muscle Protein Kinetics and Signaling during LPS-Induced Inflammation in Humans: Anticatabolic Impact of Ketone Bodies. Am. J. Clin. Nutr. 2018, 108, 857–867.
- Rodriguez, N.; Schwenk, W.F.; Beaufrere, B.; Miles, J.M.; Haymond, M.W. Trioctanoin Infusion Increases in Vivo Leucine Oxidation: A Lesson in Isotope Modeling. Am. J. Physiol. -Endocrinol. Metab. 1986, 251, E343–E348.
- Haymond, M.W.; Tessari, P.; Beaufrere, B.; Rodriguez, N.; Bailey, J.; Miles, J.M. Effects of Parenteral Lipid on Leucine Metabolism: Dependence of Fatty Acid Chain Length. JPEN J. Parenter. Enter. Nutr. 1988, 12, 94S–97S.
- Lang, C.H. Elevated Plasma Free Fatty Acids Decrease Basal Protein Synthesis, but Not the Anabolic Effect of Leucine, in Skeletal Muscle. Am. J. Physiol. -Endocrinol. Metab. 2006, 291, E666–E674.
- Tsintzas, K.; Jones, R.; Pabla, P.; Mallinson, J.; Barrett, D.A.; Kim, D.-H.; Cooper, S.; Davies, A.; Taylor, T.; Chee, C.; et al. Effect of Acute and Short-Term Dietary Fat Ingestion on Postprandial Skeletal Muscle Protein Synthesis Rates in Middle-Aged, Overweight, and Obese Men. Am. J. Physiol. -Endocrinol. Metab. 2020, 318, E417–E429.
- Furst, T.; Massaro, A.; Miller, C.; Williams, B.T.; LaMacchia, Z.M.; Horvath, P.J. β-Alanine Supplementation Increased Physical Performance and Improved Executive Function Following Endurance Exercise in Middle Aged Individuals. J. Int. Soc. Sports Nutr. 2018, 15, 32.
- Artioli, G.G.; Gualano, B.; Smith, A.; Stout, J.; Lancha, A.H. Role of β-Alanine Supplementation on Muscle Carnosine and Exercise Performance. Med. Sci. Sports Exerc. 2010, 42, 1162–1173.
- Parise, G.; Mihic, S.; MacLennan, D.; Yarasheski, K.E.; Tarnopolsky, M.A. Effects of Acute Creatine Monohydrate Supplementation on Leucine Kinetics and Mixed-Muscle Protein Synthesis. J. Appl. Physiol. 2001, 91, 1041–1047.
- Tipton, K.D.; Ferrando, A.A. Improving Muscle Mass: Response of Muscle Metabolism to Exercise, Nutrition and Anabolic Agents. Essays Biochem. 2008, 44, 85–98.
- Tessari, P.; Inchiostro, S.; Biolo, G.; Marescotti, M.C.; Fantin, G.; Boscarato, M.T.; Merola, G.; Mantero, F.; Tiengo, A. Leucine Kinetics and the Effects of Hyperinsulinemia in Patients With Cushing’s Syndrome. J. Clin. Endocrinol. Metab. 1989, 68, 256–262.
- Horber, F.F.; Haymond, M.W. Human Growth Hormone Prevents the Protein Catabolic Side Effects of Prednisone in Humans. J. Clin. Investig. 1990, 86, 265–272.
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J.P. Glucocorticoid-Induced Skeletal Muscle Atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172.
- Louard, R.J.; Bhushan, R.; Gelfand, R.A.; Barrett, E.J.; Sherwin, R.S. Glucocorticoids Antagonize Insulin’s Antiproteolytic Action on Skeletal Muscle in Humans. J. Clin. Endocrinol. Metab. 1994, 79, 278–284.
- Fryburg, D.A.; Gelfand, R.A.; Barrett, E.J. Growth Hormone Acutely Stimulates Forearm Muscle Protein Synthesis in Normal Humans. Am. J. Physiol. -Endocrinol. Metab. 1991, 260, E499–E504.
- Haymond, M.W.; Horber, F.; De Feo, P.; Kahn, S.E.; Mauras, N. Effect of Human Growth Hormone and Insulin-Like Growth Factor I on Whole-Body Leucine and Estimates of Protein Metabolism. Horm. Res. 1993, 40, 92–94.
- Kandalla, P.K.; Goldspink, G.; Butler-Browne, G.; Mouly, V. Mechano Growth Factor E Peptide (MGF-E), Derived from an Isoform of IGF-1, Activates Human Muscle Progenitor Cells and Induces an Increase in Their Fusion Potential at Different Ages. Mech. Ageing Dev. 2011, 132, 154–162.
- Górecki, D.C.; Beręsewicz, M.; Zabłocka, B. Neuroprotective Effects of Short Peptides Derived from the Insulin-like Growth Factor 1. Neurochem. Int. 2007, 51, 451–458.
- Matthews, D.E.; Pesola, G.; Campbell, R.G. Effect of epinephrine on amino acid and energy metabolism in humans. Am. J. Physiol. 1990, 258 Pt 1, E948–E956.
- Schiefermeier, M.; Ratheiser, K.; Zauner, C.; Roth, E.; Eichler, H.; Matthews, D. Epinephrine Does Not Impair Utilization of Exogenous Amino Acids in Humans. Am. J. Clin. Nutr. 1997, 65, 1765–1773.
- Kraenzlin, M.E.; Keller, U.; Keller, A.; Thélin, A.; Arnaud, M.J.; Stauffacher, W. Elevation of Plasma Epinephrine Concentrations Inhibits Proteolysis and Leucine Oxidation in Man via Beta-Adrenergic Mechanisms. J. Clin. Investig. 1989, 84, 388–393.
- Yoh, K.; Ikeda, K.; Horie, K.; Inoue, S. Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function. Int. J. Mol. Sci. 2023, 24, 1853.
- Boland, R.; Vasconsuelo, A.; Milanesi, L.; Ronda, A.C.; de Boland, A.R. 17beta-Estradiol Signaling in Skeletal Muscle Cells and Its Relationship to Apoptosis. Steroids 2008, 73, 859–863.
- Tsai, W.-J.A.; McCormick, K.M.; Brazeau, D.A.; Brazeau, G.A. Estrogen Effects on Skeletal Muscle Insulin-like Growth Factor 1 and Myostatin in Ovariectomized Rats. Exp. Biol. Med. 2007, 232, 1314–1325.
- Cho, E.-J.; Choi, Y.; Jung, S.-J.; Kwak, H.-B. Role of Exercise in Estrogen Deficiency-Induced Sarcopenia. J. Exerc. Rehabil. 2022, 18, 2–9.
- Smith, G.I.; Yoshino, J.; Reeds, D.N.; Bradley, D.; Burrows, R.E.; Heisey, H.D.; Moseley, A.C.; Mittendorfer, B. Testosterone and Progesterone, But Not Estradiol, Stimulate Muscle Protein Synthesis in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2014, 99, 256–265.
- Biolo, G.; Tipton, K.D.; Klein, S.; Wolfe, R.R. An Abundant Supply of Amino Acids Enhances the Metabolic Effect of Exercise on Muscle Protein. Am. J. Physiol. -Endocrinol. Metab. 1997, 273, E122–E129.
- Norton, L.E.; Layman, D.K. Leucine Regulates Translation Initiation of Protein Synthesis in Skeletal Muscle after Exercise. J. Nutr. 2006, 136, 533S–537S.
- Herman, J.R.; Rana, S.R.; Chleboun, G.S.; Gilders, R.M.; Hageman, F.C.; Hikida, R.S.; Kushnick, M.R.; Ragg, K.E.; Staron, R.S.; Toma, K. Correlation Between Muscle Fiber Cross-Sectional Area And Strength Gain Using Three Different Resistance-Training Programs In College-Aged Women. J. Strength Cond. Res. 2010, 24, 1.
- Jones, E.J.; Bishop, P.A.; Woods, A.K.; Green, J.M. Cross-Sectional Area and Muscular Strength: A Brief Review. Sports Med. 2008, 38, 987–994.
- Schoenfeld, B.J. The Mechanisms of Muscle Hypertrophy and Their Application to Resistance Training. J. Strength Cond. Res. 2010, 24, 2857–2872.
- Kumar, V.; Selby, A.; Rankin, D.; Patel, R.; Atherton, P.; Hildebrandt, W.; Williams, J.; Smith, K.; Seynnes, O.; Hiscock, N.; et al. Age-Related Differences in the Dose-Response Relationship of Muscle Protein Synthesis to Resistance Exercise in Young and Old Men: Age-Related Effects of Exercise on Muscle Anabolism. J. Physiol. 2009, 587, 211–217.
- Mayhew, D.L.; Kim, J.; Cross, J.M.; Ferrando, A.A.; Bamman, M.M. Translational Signaling Responses Preceding Resistance Training-Mediated Myofiber Hypertrophy in Young and Old Humans. J. Appl. Physiol. 2009, 107, 1655–1662.
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed Muscle Protein Synthesis and Breakdown after Resistance Exercise in Humans. Am. J. Physiol. -Endocrinol. Metab. 1997, 273, E99–E107.
- Yarasheski, K.E.; Pak-Loduca, J.; Hasten, D.L.; Obert, K.A.; Brown, M.B.; Sinacore, D.R. Resistance Exercise Training Increases Mixed Muscle Protein Synthesis Rate in Frail Women and Men >/=76 Yr Old. Am. J. Physiol. 1999, 277, E118–E125.
- Yarasheski, K.E.; Zachwieja, J.J.; Bier, D.M. Acute Effects of Resistance Exercise on Muscle Protein Synthesis Rate in Young and Elderly Men and Women. Am. J. Physiol. 1993, 265, E210–E214.
- Sheffield-Moore, M.; Paddon-Jones, D.; Sanford, A.P.; Rosenblatt, J.I.; Matlock, A.G.; Cree, M.G.; Wolfe, R.R. Mixed Muscle and Hepatic Derived Plasma Protein Metabolism Is Differentially Regulated in Older and Younger Men Following Resistance Exercise. Am. J. Physiol. -Endocrinol. Metab. 2005, 288, E922–E929.
- Küüsmaa, M.; Schumann, M.; Sedliak, M.; Kraemer, W.J.; Newton, R.U.; Malinen, J.-P.; Nyman, K.; Häkkinen, A.; Häkkinen, K. Effects of Morning versus Evening Combined Strength and Endurance Training on Physical Performance, Muscle Hypertrophy, and Serum Hormone Concentrations. Appl. Physiol. Nutr. Metab. 2016, 41, 1285–1294.
- Biolo, G.; Williams, B.D.; Fleming, R.Y.; Wolfe, R.R. Insulin Action on Muscle Protein Kinetics and Amino Acid Transport during Recovery after Resistance Exercise. Diabetes 1999, 48, 949–957.
