You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by LILIA ANDRIANI.
Ceftaroline fosamil is an N-phosphono prodrug of the fifth generation cephalosporin derivative ceftaroline, presenting two amino side groups located at positions 3 and 7, respectively.
- Gram-positive pathogens
- novel anti-MRSA β-lactams
- lipoglycopeptides
1. Introduction
The emergence of antibiotic-resistant Gram-positive pathogens has made a shift in antibiotic usage policies necessary, prompting the exploration of novel molecules that can effectively overcome the spread of multiple mechanisms of resistance.
Clinical expectations from evidence on antibiotics against Gram-positive bacteria must be diversified according to their community-acquired or hospital-acquired nature; this includes the need for oral antibiotics that are effective against penicillin-resistant Streptococcus pneumoniae and methicillin-resistant Staphylococcus aureus (MRSA) for community-acquired infections such as respiratory and skin/soft-tissue infections. Otherwise, more effective antibiotics are needed for serious hospital-acquired infections such as bacteremia, ventilator-associated pneumonia, and endocarditis sustained by MRSA and vancomycin-resistant enterococci (VRE). The primary outcome of interest is all-cause mortality, with other outcomes including duration of hospital stay, resource use, adverse events, and resistance development.
2. β-Lactams: Fifth Generation Cephalosporins
2.1. Ceftaroline
2.1.1. Chemical Structure and Mechanism of Action
The chemical structure of ceftaroline is depicted in Figure 1. Ceftaroline fosamil is an N-phosphono prodrug of the fifth generation cephalosporin derivative ceftaroline, presenting two amino side groups located at positions 3 and 7, respectively. It is administered for the treatment of adults with acute bacterial skin and skin structure infections. Ceftaroline fosamil is quickly hydrolysed to its active form, ceftaroline, via plasma phosphatases. Then, ceftaroline binds to penicillin-binding proteins (PBPs), particularly PBP 2A and PBPs 1, 2, and 3, which are located on the inner membrane of the bacterial cell wall, and inactivates them. PBPs are enzymes involved in the periplasmic and membrane steps of peptidoglycan biosynthesis, the main component of the bacterial cell wall. Therefore, the inactivation of PBPs affects the cross-linkage of peptidoglycan chains, thus weakening the bacterial cell wall and cell lysis [13][1].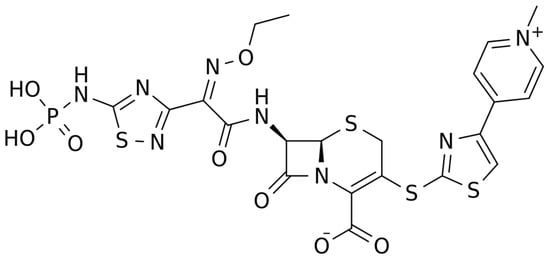
Figure 1.
Chemical structure depiction of ceftaroline fosamil.
2.1.2. Microbiological Target
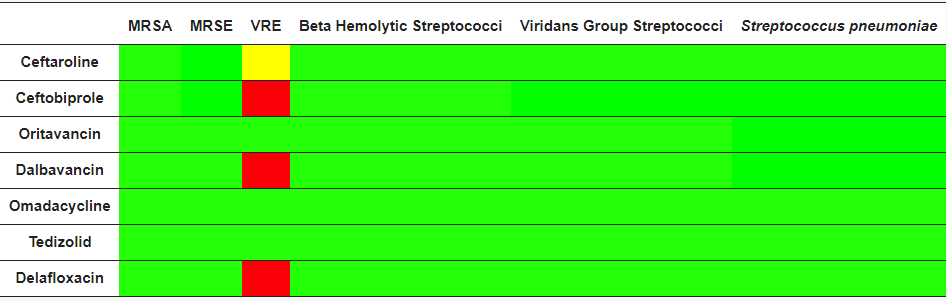
The spectrum of activity for ceftaroline includes major pathogens found in acute bacterial skin and skin structure infections (ABSSSI) and community-acquired bacterial pneumonia (CABP). Ceftaroline is a bactericidal antibiotic with a high affinity for specific penicillin-binding-proteins (PBPs) responsible for methicillin resistance in staphylococci (PBP2a) and non-sensitivity to penicillins in pneumococci (PBP 2x/2b) [14][2] (Table 1). It shows broad-spectrum activity against a range of Gram-positive bacteria, including methicillin-susceptible S. aureus (MSSA) and MRSA, vancomycin-intermediate S. aureus (VISA), heteroresistant VISA (h-VISA), vancomycin-resistant S. aureus (VRSA), daptomycin non-susceptible and linezolid-resistant S. aureus, S. pyogenes, S. agalactiae, and S. pneumoniae (including MDR strains), while its activity against E. faecalis is modest (not active against E. faecium) [15,16][3][4]. It shows activity for respiratory community-acquired Gram-negative pathogens such as Haemophilus influenzae and M. catarrhalis, including non-extended spectrum beta-lactamase producing Enterobacterales [17][5]. It has limited activity against most non-fermenting Gram-negative rods (i.e., Pseudomonas aeruginosa, Acinetobacter spp.) and many anaerobic species. Its activity against Gram-positive anaerobes, including Peptostreptococcus spp., Propionibacterium spp. and Clostridium spp., is similar to that of amoxicillin-clavulanate and 4–8 times that of ceftriaxone [18][6]. Development of resistance to ceftaroline occurs rarely in Gram-positive bacteria and at a similar rate to that of other oxyimino-cephalosporins in Gram-negative bacteria.Table 1. Microbiological targets, Green = antimicrobial activity, red = no antimicrobial activity, yellow = partial antimicrobial activity, MRSA: methicillin-resistant S. aureus; MRSE: methicillin-resistant S. epidermidis; VRE: vancomicin-resistant Enterococcus spp.
2.1.3. Clinical Use
Ceftaroline has been approved by the EMA and FDA for the treatment of adults and children with CABP and ABSSSI. Pivotal clinical trials demonstrated non-inferiority to ceftriaxone in CAP and to aztreonam + vancomycin in cSSTI [19,20,21,22][7][8][9][10]. In real life, ceftaroline is an appealing option in patients with bacteremic MRSA pneumonia, with the advantages of hydrophily, good ELF penetration, and lower nephrotoxicity compared to vancomycin [23][11]. Its empirical use in monotherapy in nosocomial pneumonia is risky given its poor activity against Gram-negatives, especially Pseudomonas [24][12]. Ceftaroline has been successfully used as an add-on to daptomycin for persistent MRSA bacteremia [25,26][13][14]. Most off-label use is for these (in order): bacteremia, endocarditis, osteoarticular infections, HAP, and meningitis [27][15]. Animal studies showed a moderate CNS penetration with better antimicrobial activity compared to ceftriaxone + vancomycin in murine pneumococcal meningitis [28][16]. In human endocarditis and meningitis, ceftaroline is commonly used at higher doses (600 mg every 8 h) [27][15]. Encephalopathy [29][17] and neutropenia [30][18] are especially reported in patients with renal failure or with long therapies, respectively.2.1.4. PK/PD Characteristics
Ceftaroline is a fifth generation cephalosporin characterized by a linear PK profile following an IV infusion, with Cmax AUC values increasing in proportion to dose increases within the range of 50–1000 mg. The median steady-state volume of ceftaroline distribution is 20.3 L, and the average binding of ceftaroline to human plasma proteins is 20% (Table 2) [28,29,30][16][17][18]. Ceftaroline was found to be predominantly eliminated by glomerular filtration (90%). The mean half-life is 2.6 h in subjects with normal renal function, eventually increasing to 6 h in patients with ESRD [31,32,33][19][20][21]. Drug dosage adjustments have been established for patients with moderate to severe renal impairment. When compared with the PK parameters assessed in healthy younger adults aged 18–45 years, healthy elderly subjects (≥65 years) showed modest alterations, likely due to naturally occurring decreases in renal function over time. Chauzy et al. recently showed that ceftaroline clearance increased non-linearly in patients with augmented renal clearance (ARC), with a reduced probability of reaching PK/PD targets, especially in patients with MRSA infections [Therefore, higher than conventional doses should be considered in patients with ARC]. Studies in patients and healthy volunteers have reported good distributions of ceftaroline in the muscles (50%), subcutaneous tissues (50%), and in the epithelial lying fluid (22%); lower drug penetration was reported in the bone tissues (10%) and in CSF (6%) (reviewed in [34][22]). The PK/PD index that is associated with efficacy for ceftaroline is the percentage of time that free drug concentrations, usually measured in serum/plasma, are above the bacteria MIC during a dosing interval, (fT > MIC). When this index was assessed for ceftaroline in murine models, median fT > MIC values of 36% and 44% achieved a 1-log kill for S. aureus and S. pneumoniae, respectively.Table 2.
Summary of the main PK/PD characteristics of the novel antibiotics for the treatment of Gram-positive bacterial infections.
| Drug | Half-Life h * |
Vd L |
Protein Binding % |
Renal Elimination % |
ELF/Plasma Ratio, % ^ |
CSF/Plasma Ratio, % ^ |
TDM in Routine |
Clinical PK/PD Efficacy Target |
Clinical PK Safety Target |
|---|---|---|---|---|---|---|---|---|---|
| Ceftaroline | 2.6 | 20 | 20% | 90% | 22% | 6% | Yes | 45–100% fT > MIC | n.e. |
| Ceftobiprole | 3.0 | 18 | 16% | 90% | 26% | 2–16% | Yes | 60–100% fT > MIC | n.e. |
| Dalbavancin | 250 | 30 | >90% | 40% | 36% | 2% | Yes | Cmin > 4 (8) mg/L | n.e. |
| Oritavancin | 300 | 80 | 85% | >90% | 5% | 1–5% | n.e. | n.e. | n.e. |
| Omadacyclin | 16 | 200 | 21% | 30% | 147% | n.e. | n.e. | n.e | n.e. |
| Tedizolid | 12 | 80 | 80% | <20% | 300% | 3–55% | n.e. | n.e. | Cmin < 0.55 mg/L |
| Delafloxacin | 12 | 30 | 85% | 50% | n.e | n.e. | n.e | n.e. | n.e. |
Vd: volume of distribution, BP: protein binding, TDM: therapeutic drug monitoring; n.e.: not established; PK/PD: pharmacokinetic/pharmadynamic%fT > MIC: % of time between 2 administrations that free drug concentrations are above the minimum inhibitory concentration; Cmin: trough concentrations; ELF: epithelial lying fluid; CSF: cerebrospinal fluid. * in subjects with normal renal function; ^ based on data from experimental studies and/or clinical observations.
2.1.5. Potential Role of TDM
Liquid chromatography methods coupled with mass–mass spectrometry (LC-MS/MS) or with UV detection (HPLC-UV) have been validated for the TDM of ceftaroline [35,36][23][24]. The clinical PK/PD targets for cephalosporin efficacy are set at 45–100%fT > MIC, based on the severity of the infection (MRSA vs. MSSA) and on the patients’ conditions (more aggressive targets should be considered in ICU settings) [37][25]. No upper safety thresholds for ceftaroline Cmin concentrations have been identified yet.2.2. Ceftobiprole
2.2.1. Chemical Structure and Mechanism of Action
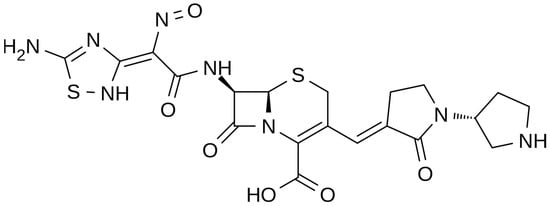
The chemical structure of ceftobiprole is depicted in Figure 2. Ceftobiprole is a fifth generation cephalosporin belonging to thiadiazoles and presenting two amino side groups located at positions 3 and 7, respectively. It was developed for the treatment of hospital- and community-acquired pneumonia and complicated skin infections. Ceftobiprole shows activity by binding to PBPs, inhibiting the cross-linkage of peptidoglycan chains and the formation of the bacterial cell wall, leading to cell lysis and death. This drug can bind to different PBPs detected in both Gram-negative and Gram-positive bacteria. Ceftobiprole forms a stable acyl-enzyme complex with PBP2a and PBP 2x, and the combination with the long side chain located in the PBP 2′-binding pocket increases the stability of the bond and inhibition of the enzymes [38][26].
Figure 2.
Depiction of the chemical structure of ceftobiprole.
2.2.2. Microbiological Target
Ceftobiprole is a fifth generation cephalosporin that has extended activity against a wide spectrum of Gram-negatives and Gram-positives. Concerning Gram-negatives, ceftobiprole retains activity against Pseudomonas aeruginosa, most members of the order Enterobacterales, and some anaerobic bacteria [39][27]. It maintains its stability against a wide variety of β-lactamases such as TEM and SHV types [40][28]. Ceftobiprole has major activity against MRSA, penicillin-resistant S. pneumoniae, and ampicillin-susceptible E. faecalis. In all cases, its activity is due to its high-affinity binding to the penicillin-binding proteins (PBPs), including the acquired PBP2a of MRSA strains, PBP2a of Staphylococcus epidermidis, and PBP2x of penicillin-resistant S. pneumoniae, resulting in the blocking of wall synthesis and bacterial death [41][29]. Ceftobiprole is degraded by acquired extended-spectrum β-lactamases (ESBLs) and carbapenemases (both serine-carbapenemases and metallo-carbapenemases), and it is also degraded by some class D enzymes. Ceftobiprole does not bind to PBP5; therefore, it is not active on E. faecium. The possibility of acquired resistance to ceftobiprole appears to be low; multiple genes and pathways may be potentially involved but are yet to be precisely defined [39,42][27][30].2.2.3. Clinical Use
Ceftobiprole has been approved by major European countries and several non-European countries (excluding the US) for CAP and HAP. Pivotal studies demonstrated non-inferiority to ceftriaxone (+optional linezolid if MRSA suspected) for CAP [43][31] and to ceftazidime + linezolid for HAP (while failing to reach non-inferiority for VAP) [44][32]. In 2021, a phase 3 trial demonstrated non-inferiority of ceftobiprole to vancomycin + aztreonam in ABSSSI [45][33]. From a clinical point of view, the potential of ceftobiprole is similar to that of ceftaroline, where ceftaroline has lower MICs for Staphylococcus while ceftobiprole has lower MICs for Enterobacterales with a degree of stability to AmpC enzymes but not ESBL [46][34]. In real life, ceftobiprole is commonly used for endocarditis (42%), bone and joint infection (24%), and HAP (15%), proving that most of its use is off label [47][35]. Good cure rates in patients with endocarditis, who were treated with daptomycin and ceftobiprole in combination, have also been reported [48][36]. From a safety point of view, ceftobiprole is expected to be a weak inducer of a Clostridioides difficile infection compared to other cephalosporins [49][37]. Its safety profile appears good, but data still need to be more conclusive.2.2.4. PK/PD Characteristics
Ceftobiprole is a fifth generation cephalosporin administered via IV as a prodrug (ceftobiprole medocaril) and rapidly converted by plasma esterases into its active form. This cephalosporin presents linear PK, with an elimination half-life of about 3 h, a low protein binding (16%), and a volume of distribution of 18–20 L [50,51,52][38][39][40]. Ceftobiprole has a limited metabolism, and its major route of elimination is through glomerular filtration. Accordingly, drug clearance is reduced in patients with impaired renal function, and drug dose reduction based on creatinine clearance has been established in this clinical setting [52][40]. Conversely, prolonged drug infusions have been suggested for patients with ARC to optimize ceftobiprole exposure [52][40]. No differences in ceftobiprole PK were found in patients with or without ECMO [53][41]. Studies in healthy volunteers have reported a good penetration of ceftobiprole in the muscles (69%), adipose tissues (49%) and in epithelial lying fluid (26%); lower drug penetration was reported in bone tissues (22%) [54,55,56,57][42][43][44][45]. Similar to other cephalosporins, ceftobiprole shows time-dependent antibacterial activity. A recent population pharmacokinetic/pharmacodynamic analysis demonstrated that at the standard ceftobiprole dose and at a pharmacodynamic target of 60% t > MIC of the dosing interval, more than 90% of the population was adequately exposed to the drug [58][46].2.2.5. Potential Role of TDM
LC-MS/MS and HPLC-UV methods have been validated for the TDM of ceftobiprole [58,59,60,61][46][47][48][49]. In a case report, the clinical PK/PD target for ceftobiprole efficacy was set at 100%fT > 4xMIC for the treatment of an ICU patient undergoing continuous renal replacement therapy [62][50].References
- Kaushik, D.; Rathi, S.; Jain, A. Ceftaroline: A comprehensive update. Int. J. Antimicrob. Agents 2011, 37, 389–395.
- Biek, D.; Critchley, I.A.; Riccobene, T.A.; Thye, D.A. Ceftaroline fosamil: A novel broad-spectrum cephalosporin with expanded anti-Grampositive activity. J. Antimicrob. Chemother. 2010, 65 (Suppl. 4), iv9–iv16.
- Lodise, T.P.; Low, D.E. Ceftaroline fosamil in the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections. Drugs 2012, 72, 1473–1493.
- Zhanel, G.G.; Calic, D.; Schweizer, F.; Zelenitsky, S.; Adam, H.; Lagacé-Wiens, P.R.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; Karlowsky, J.A. New lipoglycopeptides: A comparative review of dalbavancin, oritavancin and telavancin. Drugs 2010, 70, 859–886.
- Casapao, A.M.; Steed, M.E.; Levine, D.P.; Rybak, M.J. Ceftaroline fosamil for community-acquired bacterial pneumonia and acute bacterial skin and skin structure infection. Expert Opin. Pharmacother. 2012, 13, 1177–1186.
- Citron, D.M.; Tyrrell, K.L.; Merriam, C.V.; Goldstein, E.J. In vitro activity of ceftaroline against 623 diverse strains of anaerobic bacteria. Antimicrob. Agents Chemother. 2010, 54, 1627–1632.
- File, T.M., Jr.; Low, D.E.; Eckburg, P.B.; Talbot, G.H.; Friedland, H.D.; Lee, J.; Llorens, L.; Critchley, I.A.; Thye, D.A. FOCUS 1: A randomized, double-blinded, multicentre, Phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J. Antimicrob. Chemother. 2011, 66, 19–32.
- Low, D.E.; File, T.M., Jr.; Eckburg, P.B.; Talbot, G.H.; Friedland, H.D.; Lee, J.; Llorens, L.; Critchley, I.A.; Thye, D.A.; Focus 2 Investigators; et al. FOCUS 2: A randomized, double-blinded, multicentre, Phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J. Antimicrob. Chemother. 2011, 66, 33–44.
- Corey, G.R.; Wilcox, M.H.; Talbot, G.H.; Thye, D.; Friedland, H.D.; Baculik, T.; CANVAS 1 investigators; Mehra, P.; Alpert, M.; Baird, I.; et al. CANVAS 1: The first Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J. Antimicrob. Chemother. 2010, 65, 41–51.
- Wilcox, M.H.; Corey, G.R.; Talbot, G.H.; Thye, D.; Friedland, H.D.; Baculik, T.; CANVAS 2 investigators; Manos, P.; Lee, P.; Bush, L.; et al. CANVAS 2: The second Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J. Antimicrob. Chemother. 2010, 65, 53–65.
- Vazquez, J.A.; Maggiore, C.R.; Cole, P.; Smith, A.; Jandourek, A.; Friedland, H.D. Ceftaroline Fosamil for the Treatment of Bacteremia Secondary to Acute Bacterial Skin and Skin Structure Infections or Community-Acquired Bacterial Pneumonia. Infect. Dis. Clin. Pract. 2015, 23, 39–43.
- Zhang, H.; Xu, Y.; Jia, P.; Zhu, Y.; Zhang, G.; Zhang, J.; Duan, S.; Kang, W.; Wang, T.; Jing, R.; et al. Global trends of antimicrobial susceptibility to ceftaroline and ceftazidime-avibactam: A surveillance study from the ATLAS program (2012–2016). Antimicrob. Resist. Infect. Control 2020, 9, 166.
- Geriak, M.; Haddad, F.; Rizvi, K.; Rose, W.; Kullar, R.; LaPlante, K.; Yu, M.; Vasina, L.; Ouellette, K.; Zervos, M.; et al. Clinical Data on Daptomycin plus Ceftaroline versus Standard of Care Monotherapy in the Treatment of Methicillin-Resistant Staphylococcus aureus Bacteremia. Antimicrob. Agents Chemother. 2019, 63, e02483-18.
- Huang, C.; Chen, I.; Lin, L. Comparing the Outcomes of Ceftaroline plus Vancomycin or Daptomycin Combination Therapy versus Vancomycin or Daptomycin Monotherapy in Adults with Methicillin-Resistant Bacteremia—A Meta-Analysis. Antibiotics 2022, 11, 1104.
- Pani, A.; Colombo, F.; Agnelli, F.; Frantellizzi, V.; Baratta, F.; Pastori, D.; Scaglione, F. Off-label use of ceftaroline fosamil: A systematic review. Int. J. Antimicrob. Agents 2019, 54, 562–571.
- Cottagnoud, P.; Cottagnoud, M.; Acosta, F.; Stucki, A. Efficacy of ceftaroline fosamil against penicillin-sensitive and -resistant Streptococcus pneumoniae in an experimental rabbit meningitis model. Antimicrob. Agents Chemother. 2013, 57, 4653–4655.
- Martin, T.C.S.; Chow, S.; Johns, S.T.; Mehta, S.R. Ceftaroline-associated Encephalopathy in Patients with Severe Renal Impairment. Clin. Infect. Dis. 2020, 70, 2002–2004.
- Sullivan, E.L.; Turner, R.B.; O’Neal, H.R., Jr.; Crum-Cianflone, N.F. Ceftaroline-Associated Neutropenia: Case Series and Literature Review of Incidence, Risk Factors, and Outcomes. Open Forum Infect. Dis. 2019, 6, ofz168.
- Kiang, T.K.; Wilby, K.J.; Ensom, M.H. A critical review on the clinical pharmacokinetics, pharmacodynamics, and clinical trials of ceftaroline. Clin. Pharmacokinet. 2015, 54, 915–931.
- Merker, A.; Danziger, L.H.; Rodvold, K.A.; Glowacki, R.C. Pharmacokinetic and pharmacodynamic evaluation of ceftaroline fosamil. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1741–1750.
- Riccobene, T.; Jakate, A.; Rank, D. A series of pharmacokinetic studies of ceftaroline fosamil in select populations: Normal subjects, healthy elderly subjects, and subjects with renal impairment or end-stage renal disease requiring hemodialysis. J. Clin. Pharmacol. 2014, 54, 742–752.
- Chauzy, A.; Gregoire, N.; Ferrandière, M.; Lasocki, S.; Ashenoune, K.; Seguin, P.; Boisson, M.; Couet, W.; Marchand, S.; Mimoz, O.; et al. Population pharmacokinetic/pharmacodynamic study suggests continuous infusion of ceftaroline daily dose in ventilated critical care patients with early-onset pneumonia and augmented renal clearance. J. Antimicrob. Chemother. 2022, 77, 3173–3179.
- Alarcia-Lacalle, A.; Barrasa, H.; Maynar, J.; Canut-Blasco, A.; Gómez-González, C.; Solinís, M.Á.; Isla, A.; Rodríguez-Gascón, A. Quantification of Ceftaroline in Human Plasma Using High-Performance Liquid Chromatography with Ultraviolet Detection: Application to Pharmacokinetic Studies. Pharmaceutics 2021, 13, 959.
- Grégoire, M.; Leroy, A.G.; Bouquié, R.; Malandain, D.; Dailly, E.; Boutoille, D.; Renaud, C.; Jolliet, P.; Caillon, J.; Deslandes, G. Simultaneous determination of ceftaroline, daptomycin, linezolid and rifampicin concentrations in human plasma by on-line solid phase extraction coupled to high-performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2016, 118, 17–26.
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153.
- Dauner, D.G.; Nelson, R.E.; Taketa, D.C. Ceftobiprole: A novel, broad-spectrum cephalosporin with activity against methicillin-resistant Staphylococcus aureus. Am. J. Health Syst. Pharm. 2010, 67, 983–993.
- Morosini, M.I.; Díez-Aguilar, M.; Cantón, R. Mechanisms of action and antimicrobial activity of ceftobiprole. Rev. Esp. Quimioter. 2019, 32, 3–10.
- Queenan, A.M.; Shang, W.; Kania, M.; Malcolm, G.P.; Bush, K. Interactions of Ceftobiprole with β-Lactamases from Molecular Classes A to D. Antimicrob. Agents Chemother. 2007, 51, 3089–3095.
- Giacobbe, D.R.; De Rosa, F.G.; Tascini, C.; Tumbarello, M.; Viale, P.; Bassetti, M. Ceftobiprole: Drug evaluation and place in therapy. Expert Rev. Anti Infect. Ther. 2019, 17, 689–698.
- Mlynarczyk Bonikowska, B.; Kowalewski, C.; Krolak-Ulinska, A.; Marusza, W. Molecular Mechanisms of Drug Resistance in Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 8088.
- Nicholson, S.C.; Welte, T.; File, T.M., Jr.; Strauss, R.S.; Michiels, B.; Kaul, P.; Balis, D.; Arbit, D.; Amsler, K.; Noel, G.J. A randomised, double-blind trial comparing ceftobiprole medocaril with ceftriaxone with or without linezolid for the treatment of patients with community-acquired pneumonia requiring hospitalisation. Int. J. Antimicrob. Agents 2012, 39, 240–246.
- Awad, S.S.; Rodriguez, A.H.; Chuang, Y.C.; Marjanek, Z.; Pareigis, A.J.; Reis, G.; Scheeren, T.W.L.; Sánchez, A.S.; Zhou, X.; Saulay, M.; et al. A phase 3 randomized double-blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital-acquired pneumonia. Clin. Infect. Dis. 2014, 59, 51–61.
- Overcash, J.S.; Kim, C.; Keech, R.; Gumenchuk, I.; Ninov, B.; Gonzalez-Rojas, Y.; Waters, M.; Simeonov, S.; Engelhardt, M.; Saulay, M.; et al. Ceftobiprole Compared with Vancomycin Plus Aztreonam in the Treatment of Acute Bacterial Skin and Skin Structure Infections: Results of a Phase 3, Randomized, Double-blind Trial (TARGET). Clin. Infect. Dis. 2021, 73, e1507–e1517.
- Livermore, D.M.; Hope, R.; Brick, G.; Lillie, M.; Reynolds, R. BSAC Working Parties on Resistance Surveillance. Non-susceptibility trends among Enterobacteriaceae from bacteraemia in the UK and Ireland, 2001–2006. J. Antimicrob. Chemother. 2008, 62, ii41–ii54.
- Zhanel, G.G.; Kosar, J.; Baxter, M.; Dhami, R.; Borgia, S.; Irfan, N.; MacDonald, K.S.; Dow, G.; Lagacé-Wiens, P.; Dube, M.; et al. Real-life experience with ceftobiprole in Canada: Results from the CLEAR (CanadianLEadership onAntimicrobialReal-life usage) registry. J. Glob. Antimicrob. Resist. 2021, 24, 335–339.
- Tascini, C.; Attanasio, V.; Ripa, M.; Carozza, A.; Pallotto, C.; Bernardo, M.; Francisci, D.; Oltolini, C.; Palmiero, G.; Scarpellini, P. Ceftobiprole for the treatment of infective endocarditis: A case series. J. Glob. Antimicrob. Resist. 2020, 20, 56–59.
- Bäckström, T.; Panagiotidis, G.; Beck, O.; Asker-Hagelberg, C.; Rashid, M.U.; Weintraub, A.; Nord, C.E. Effect of ceftobiprole on the normal human intestinal microflora. Int. J. Antimicrob. Agents 2010, 36, 537–541.
- Murthy, B.; Hoffmann-Schmitt, A. Pharmacokinetics and pharmacodynamics of ceftobiprole, an anti-MRSA cephalosporin with broad-spectrum activity. Clin. Pharmacokinet. 2008, 47, 21–33.
- Horn, K.S.; Danziger, L.H.; Rodvold, K.A.; Glowacki, R.C. Pharmacokinetic drug evaluation of ceftobiprole for the treatment of MRSA. Expert Opin. Drug Metab. Toxicol. 2017, 13, 463–472.
- Torres, A.; Mouton, J.W.; Pea, F. Pharmacokinetics and Dosing of Ceftobiprole Medocaril for the Treatment of Hospital- and Community-Acquired Pneumonia in Different Patient Populations. Clin. Pharmacokinet. 2016, 55, 1507–1520.
- Coppens, A.; Zahr, N.; Chommeloux, J.; Bleibtreu, A.; Hekimian, G.; Pineton de Chambrun, M.; LeFevre, L.; Schmidt, M.; Robert, J.; Junot, H.; et al. Pharmacokinetics/pharmacodynamics of ceftobiprole in patients on extracorporeal membrane oxygenation. Int. J. Antimicrob. Agents 2023, 61, 106765.
- Barbour, A.; Schmidt, S.; Sabarinath, S.N.; Grant, M.; Seubert, C.; Skee, D.; Murthy, B.; Derendorf, H. Soft-tissue penetration of ceftobiprole in healthy volunteers determined by in vivo microdialysis. Antimicrob. Agents Chemother. 2009, 53, 2773–2776.
- Landersdorfer, C.B.; Bulitta, J.B.; Kinzig, M.; Holzgrabe, U.; Sorgel, F. Penetration of antibacterials into bone: Pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin. Pharmcokinet. 2009, 48, 89–124.
- Schmitt-Hoffman, A.; Engelhardt, M.; Spickermann, J.; Jones, M.; Kaufhold, A. Bone penetration of the new-generation cephalosporin ceftobiprole in patients following hip replacement surgery . In Proceedings of the 26th Annual European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Amsterdam, The Netherlands, 9–12 April 2016.
- Rodvold, K.A.; Nicolau, D.P.; Lodise, T.P.; Khashab, M.; Noel, G.J.; Kahn, J.B.; Gotfried, M.; Murray, S.A.; Nicholson, S.; Laohavaleeson, S.; et al. Identifying exposure targets for treatment of staphylococcal pneumonia with ceftobiprole. Antimicrob. Agents Chemother. 2009, 53, 3294–3301.
- Muller, A.E.; Punt, N.; Mouton, J.W. Exposure to ceftobiprole is associated with microbiological eradication and clinical cure in patients with nosocomial pneumonia. Antimicrob. Agents Chemother. 2014, 58, 2512–2519.
- Llopis, B.; Bleibtreu, A.; Schlemmer, D.; Robidou, P.; Paccoud, O.; Tissot, N.; Noé, G.; Junot, H.; Luyt, C.É.; Funck-Brentano, C.; et al. Simple and accurate quantitative analysis of cefiderocol and ceftobiprole in human plasma using liquid chromatography-isotope dilution tandem mass spectrometry: Interest for their therapeutic drug monitoring and pharmacokinetic studies. Clin. Chem. Lab. Med. 2021, 59, 1800–1810.
- Magréault, S.; Jaureguy, F.; Zahar, J.R.; Méchaï, F.; Toinon, D.; Cohen, Y.; Carbonnelle, E.; Jullien, V. Automated HPLC-MS/MS assay for the simultaneous determination of ten plasma antibiotic concentrations. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2022, 1211, 123496.
- Lima, B.; Bodeau, S.; Quinton, M.C.; Leven, C.; Lemaire-Hurtel, A.S.; Bennis, Y. Validation and Application of an HPLC-DAD Method for Routine Therapeutic Drug Monitoring of Ceftobiprole. Antimicrob. Agents Chemother. 2019, 63, e00515-19.
- Cojutti, P.G.; Merelli, M.; De Stefanis, P.; Fregonese, C.; Lucchese, F.; Bassetti, M.; Pea, F. Disposition of ceftobiprole during continuous venous-venous hemodiafiltration (CVVHDF) in a single critically ill patient. Eur. J. Clin. Pharmacol. 2018, 74, 1671–1672.
More
