Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Shuge Dai and Version 2 by Sirius Huang.
Energy storage systems are of great importance in daily life due to our dependence on portable electronic devices and hybrid electric vehicles. Among these energy storage systems, hybrid supercapacitor devices, constructed from a battery-type positive electrode and a capacitor-type negative electrode, have attracted widespread interest due to their potential applications. In general, they have a high energy density, a long cycling life, high safety, and environmental friendliness.
- hybrid supercapacitors
- electrode materials
- design structure
- energy storage mechanism
1. Introduction
In recent years, the increasing environmental problems and energy challenges have stimulated urgent demand for developing green, efficient, and sustainable energy sources, as well as revolutionary technologies associated with energy conversion and storage systems [1][2][1,2]. Among the diverse energy storage devices, supercapacitors (SCs) have received extensive attention due to their high power density, fast charge and discharge rates, and long-term cycling stability [3][4][5][3,4,5]. Generally, SCs can be classified as electrical double-layer capacitors (EDLCs), pseudocapacitors (PCs), or hybrid supercapacitors (HSCs) depending on the energy storage mechanism [6][7][8][9][10][6,7,8,9,10]. EDLCs collect energy through the ion absorption/desorption on the electrode/electrolyte interface without the charge transfer reaction [7][8][7,8]. PCs harvest energy through fast redox reactions at or near the surface of the electrode material [3][9][3,9]. Different charge storage mechanisms occur in the electrode materials of HSCs. For example, the negative electrode utilizes the double-layer storage mechanism (activated carbon, graphene), whereas the others accumulate charge by using fast redox reactions (typically transition metal oxides and hydroxides) [11][12][13][14][11,12,13,14]. HSCs have attracted enormous attention as they can provide excellent performance with higher energy and power densities at high charge/discharge rates [12][13][12,13]. More importantly, HSCs provide an important future opportunity for energy storage devices to meet the demands of both higher energy and power densities for powering portable electronic devices, hybrid electric vehicles, and industrial equipment.
At present, nanostructured transition metal oxides, sulfides, and hydroxides [15][16][17][18][19][20][21][15,16,17,18,19,20,21] are being widely explored as positive electrodes for HSCs. Such materials display a very fast charge/discharge rate to offer high power density. Unfortunately, many battery-type electrodes, such as Ni(OH)2 [22][23][22,23] or other materials, that exhibit faradaic behavior (even those that are electrochemically irreversible) have been considered as pseudocapacitive materials in many reports, which confuses the readers [24][25][26][24,25,26]. As suggested by Gogosti et al. [10], it is inappropriate to describe nickel-based oxides, sulfides, and hydroxides as pseudocapacitive electrode materials in alkaline aqueous electrolytes because they undergo faradaic reactions, where their electrochemical signature is analogous to that of a “battery” material. Therefore, the concept of “capacitance” (F) cannot be applied to purely faradaic behavior, and “capacity” (C or mAh) is the most appropriate and meaningful metric to represent the performance of such materials [26]. In addition, some researchers may mistakenly consider the HSCs as asymmetric supercapacitors (ASCs) that are based on two different supercapacitor-type electrodes (i.e., capacitive electrodes and/or pseudocapacitive electrodes), which also aggravates the confusion for readers [27]. The definition of an ASC device is very broad since it refers to every combination of positive and negative electrodes with the same nature regardless of the difference between the two electrodes (weight, thickness, material, etc.) [7]. However, an HSC device should be used when pairing two electrodes with different charge storage behaviors, such as one capacitive and the other faradaic, and the performance of such a device is in between a supercapacitor and a battery [27]. Some researchers have presented a well-rounded view in recent literature [27][28][29][27,28,29].
2. Recent Advances in Materials for Hybrid Supercapacitors
HSCs are generally composed of three components (Figure 1): electrodes, electrolytes, and separators. The performance of HSCs is mainly determined by the electrochemical activity and kinetic features of the electrodes. To improve the energy and power density of HSCs, it is crucial to enhance the kinetics of ion and electron transport in electrodes and at the electrode/electrolyte interface [30][33]. Therefore, electrode materials, as the essential soul of the devices, play a decisive role in the performance of HSCs.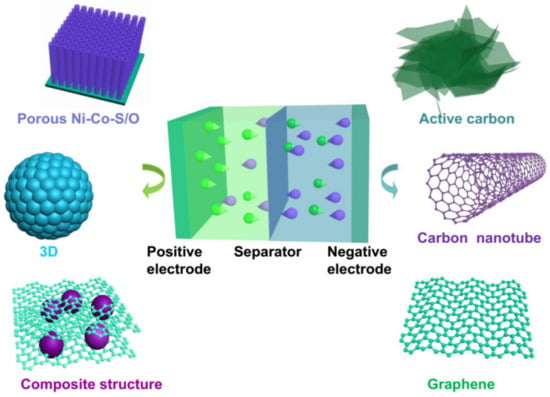
Figure 1.
Illustration of a hybrid supercapacitor system.
2.1. Positive Electrode Materials
The performance of a HSC device is mainly determined by the positive electrode materials [10]. In recent years, transition metal oxides/sulfides/hydroxides [31][34] have been considered as promising electrode materials for HSCs since they can provide a variety of oxidation states for fast surface redox reactions.2.1.1. Nickel Oxides/Hydroxides/Sulfides
Recently, Ni-based oxides/hydroxides, such as NiO [32][33][34][35][36][35,36,37,38,39] and Ni(OH)2 [37][38][39][40][41][40,41,42,43,44], have been widely reported as electrode materials for HSCs due to their attractive theoretical specific capacity and potentially high-rate capability in alkaline aqueous solutions. NiO is a promising battery-type material due to its high theoretical specific capacity (1292 C g−1 in a potential window of 0.5 V), well-defined redox behavior, and low cost [35][38]. For instance, Ren et al. [42][45] prepared honeycomb-like mesoporous NiO microspheres and revealed a high specific capacitance of 635 C g−1 at 1 A g−1. Even at 5 A g−1, it also exhibited a high specific capacity of 472.5 C g−1 with 88.4% retention after 3500 cycles, demonstrating its superior performance. Cai et al. [43][46] prepared NiO nanoparticles and found a high specific capacity of 693 C g−1 at 1 A g−1, but the rate of capability could only retain 62% (430 C g−1) as the current density increased to 50 A g−1. The poor rate performance is caused by its low electrical conductivity. Although many recent efforts have been carried out on NiO electrodes, the acquired specific capacity is usually lower than the theoretical capacity of NiO. The relatively poor conductivity of NiO limited its specific capacity, and hindered the fast electron transport required for high charge–discharge rates. Compared to NiO materials, Ni(OH)2 has been considered as a promising candidate for HSCs due to its high theoretical capacity (1041 C g−1 in a potential window of 0.5 V), excellent redox behavior, ease of synthesis, abundant sources, low cost, and environmental friendliness [44][47]. Currently, many advances have been widely reported, as summarized in Table 1. During the last decades, numerous efforts have been devoted to fabricating high-performance electrodes based on Ni(OH)2 materials for energy storage devices, but there are still some challenging issues. Owing to its low conductivity, the Faradic redox reactions can only take place on its surface, and most of the reported Ni(OH)2 materials are inaccessible to electrolyte ions and remain as dead volumes in HSCs [45][46][48,49]. In recent years, many strategies have been explored to address this issue, including the synthesis of nanoscale or porous structures (Figure 2a), atomic substitution or doping (Figure 2b), and forming a composite with carbon-based or other materials (Figure 2c) [47][48][50,51]. For instance, the as-prepared hybrid electrode (Ni(OH)2/carbonnantube/polymer) by Jiang et al. [46][49] delivered an ultrahigh specific capacity of 1631 C g−1 at 5 mV s−1, excellent rate capability (71.9% capacity retention at 100 mV s−1), and long cycle life (85% capacitance retention after 20,000 cycles). In the hybrid, the conducting polymer coating contributes to stabilizing the whole electrode by reducing the dissolution of active materials, thus greatly improving the rate capability and cycling stability of the electrode. Fabricating a composite by incorporating highly conductive graphene nanosheets into Ni(OH)2 materials is considered as the most effective strategy to enhance the intrinsic properties of Ni(OH)2. Li et al. [49][52] reported a novel Ni(OH)2/rGO hybrid material, which not only exhibited a high specific capacity (1007.5 C g−1 at 0.5 A g−1), but also showed good life cycle stability (108% capacitance retention after 8000 cycles), revealing its good performance by incorporating rGO. Guo et al. [50][53] prepared a Ni(OH)2/rGO hybrid electrode and found a high specific capacity (1388 C g−1 at 2 A g−1) and remarkable rate capability (785 C g−1 at 50 A g−1). A Ni(OH)2-porous nitrogen-doped graphene hybrid architecture was also synthesized by Aghazadeh et al. [51][54]. The composite exhibited a specific capacity of 701 C g−1 and a capacity retention of 92.8% after 7000 cycles at 10 A g−1. In addition, the electrochemical performances of Ni(OH)2/rGO composites that have been reported thus far are compared in Table 2. It clearly reveals that, despite great achievement by hybridizing with rGO, Ni(OH)2 still requires further improvements, particularly in high-rate performance as well as in long cycle life.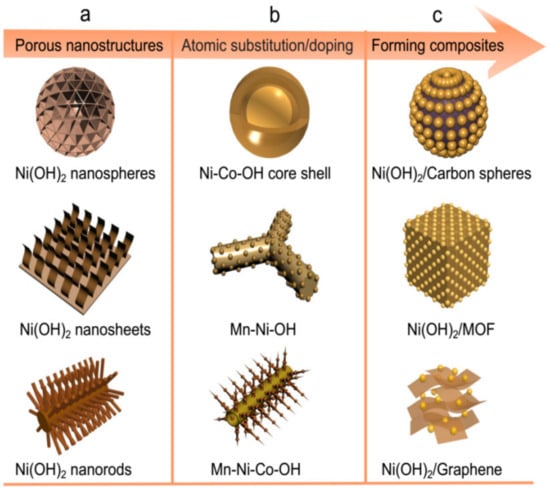
Figure 2. Illustration of nanoscale or porous structures of Ni(OH)2 (a), atomic substitution or doping (b), and fabricating a composite with carbon-based or other materials (c).
Table 1.
Specific capacity of Ni(OH)
2
electrodes.
| Electrode Materials | Electrolyte | ∆E (V)Voltage (V) |
Maximum Capacity Current Load or Scan Rate |
(C g | −1 | )Specific Capacity (C g | −1 | ) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Capacity Retention | Cycle Stability | Ref. | |||||||||
| 3D nanoporous Ni(OH)2 | 6.0 M KOH | 0–0.5 | |||||||||
| Ni(OH)2 nanoplatelets/rGO | 0.45 | 955 C g−1 (1 A g−1) | 7 A g−1 | 759.5 | [ | 58.6% (80 A g−1)52][ | 102% (5000 cycles)55] | ||||
| [ | 71 | ] | [ | 74 | ] | Ni(OH)2 nanospheres | 1.0 M KOH | 0–0.5 | 20 A g−1 | 934 | [53][56] |
| 3D Ni(OH)2/rGO network | 0.5 | 563 C g−1 (0.5 A g−1) | 61.8% (10 A g−1) | 87% (1000 cycles) | [72][75] | α-Ni(OH)2 nanobristles | 1.0 M KOH | ||||
| Ni(OH) | 0–0.45 | 2/rGO | 2 A g−1 | 940.5 | 0.6 | 941 C g−1 (4 A g−1) | 27% (11.2 A g−1)[ | 75% (1000 cycles)54][57] | |||
| [ | 73 | ] | [ | 76 | ] | Ni(OH)2 microspheres | 2.0 M KOH | 0–0.55 | 0.5 A g−1 | 704.5 | [55][58] |
| Ni(OH)2/rGO aerogel | 0.5 | 516 C g−1 (0.5 A g−1) | 54.3% (2 A g−1) | 95% (2000 cycles) | [74][77] | Mesoporous a-Ni(OH)2 | 2.0 M KOH | 0–0.55 | 0.5 A g−1 | 983.9 | |
| Ni(OH)2/3D rGO | 0.5 | 690 C g−1 (1 A g−1) | [ | 86.7% (60 A g−1) | 56][ | 78% (1000 cycles)59] | |||||
| [ | 75 | ] | [ | 78 | ] | Ni(OH)2 nanoboxes | 2.0 M KOH | 0–0.5 | |||
| Ni(OH)2 nanoparticles/rGO | 0.38 | 858 C g−1 (0.5 A g−1 | 1 A g−1 | 1247.5 | )[57 | 52.7% (10 A g−1)][60] | |||||
| 89% (1000 cycles) | [ | 76 | ] | [ | 79] | α-Ni(OH)2 nanowires | 2.0 M KOH | ||||
| Ni(OH) | 0–0.4 | 1 A g | −1 | 889.2 | 2 nanosheets/rGO[ | 0.45 | 838 C g−1 (0.8 A g−1)58] | 62.3% (6.4 A g−1)[61] | |||
| 92% (2000 cycles) | [ | 77 | ] | [ | 80] | Ni(OH)2 nanosheets | 6.0 M KOH | ||||
| Ni(OH)2 | 0–0.5 | nanocrystals/rGO | 0.5 | 951.5 C g−1 (1 A g−12 A g−1 | 825.6 | ) | 60.9% (20 A g−1)[59][62] | ||||
| 70% (1000 cycles) | [ | 78 | ] | [ | 81] | Ni(OH)2 nanoflakes | 1.0 M KOH | 0–0.4 | 1 A g−1 | 566.4 | [60][63] |
| Ni(OH)2 nanoplates/rGO | 0.5 | 667 C g−1 (2.8 A g−1) | 71% (45.7 A g−1) | 100% (2000 cycles) | [79][82] | Ni(OH)2 nanocubes | 3.0 M KOH | 0–0.45 | |||
| Ni(OH)2/rGO | 0.55 | 1206 C g−1 (2 mV s−1) | 1 A g−1 | 828.9 | [ | 41% (20 mV s−1)61][ | 95% (2000 cycles)64] | ||||
| [ | 80 | ] | [ | 83 | ] | Amorphous α-Ni(OH)2 | 2.0 M KOH | 0–0.35 | 2 A g−1 | ||
| Ni(OH) | 818.3 | 2/rGO | 0.55 | 954 C g−1 (1 mV s-1) | 30% (50 mV s−1)[62] | 88% (1000 cycles)[65] | |||||
| [ | 81 | ] | [ | 84 | ] | Cabbage-like α-Ni(OH)2 | 1.0 M KOH | 0.2–0.6 | 1 mA cm−2 | 761.2 | |
| β-Ni(OH)2/rGO | [ | 63 | ] | [ | 66] | ||||||
| 0.5 | 971 C g | −1 (1 A g-1) | 67.9% (40 A g−1) | 81% (2000 cycles) | [82][85] | Ni(OH)2 nanosheets | 2.0 M KOH | ||||
| Ni(OH)2 nanoflowers/rGO | 0–0.45 | 1 A g | −1 | 1072.9 | 0.55[ | 598 C g−1 (1 A g-1) | 58% (10 A g−1) | 95% (1000 cycles)64][67] | |||
| [ | 83 | ] | [ | 86 | ] | Ni(OH)2 platelets | 2.0 M KOH | ||||
| Flower-like Ni(OH) | 0–0.6 | 2/rGO | 0.5 A g−1 | 0.4 | 642 C g−1 (1 A g-1) | 18.7% (30 A g1160.4 | −1)[65] | 86% (2200 cycles)[68] | |||
| [ | 84 | ] | β-Ni(OH)2 nanosheets | 6.0 M KOH | 0–0.6 | 5 mV s−1 | 1041 | [66][69] | |||
| [ | 87 | ] | |||||||||
| Ni(OH)2/rGO | 0.45 | 546 C g−1 (5 mV s-1) | 35% (100 mV s−1) | 88% (1000 cycles) | [85][88] | α-Ni(OH)2 | 2.0 M KOH | 0–0.5 | 2 mV s−1 | 267 | [67][70] |
| α-Ni(OH)2 microspheres | 6.0 M KOH | 0–0.4 | 1 A g−1 | 992.3 | [68][71] | ||||||
| β-Ni(OH)2 nanosheets | 6.0 M KOH | 0–0.4 | 5 mA cm−2 | 790.3 | [69][72] | ||||||
| β-Ni(OH)2 | 6.0 M KOH | −0.05–0.35 | 1 A g−1 | 712 | [70][73] |
Table 2.
Summary of performances of Ni(OH)
2
/rGO composites.
| Electrode Materials |
|---|
2.1.2. Cobalt Oxides/Hydroxides
Among various transition metal oxides, Co3O4 has attracted wide attention for its high energy storage capacity (3560 F g−1), low cost, environmental friendliness, multiple valence sites, and high activity in water oxidation [86][87][99,100]. Electron and ion transport efficiency for charge storage in Co3O4-based pseudocapacitors mainly depends on electrode properties such as surface area, morphology, and alignment of nanocrystalline phases [88][89][101,102]. In the past decade, numerous Co3O4 nanostructures have been fabricated and tested for superior performance in the field of energy storage [90][91][92][93][94][95][96][97][103,104,105,106,107,108,109,110]. For example, Yang et al. [96][109] synthesized Pr-doped Co3O4 nanoflakes, which exhibited a high specific capacity of 640 C g−1 at a current density of 2 A g−1, and 64% of the capacity retention at a high current density of 10 A g−1. Zhang et al. [97][110] fabricated the Cl-doped Co3O4 hierarchical nanospheres and observed significant performance with an ultrahigh specific capacity of 814 C g−1 at a current density of 1 A g−1, high-rate capability (63.2% capacity retention at 32 A g−1) and good cycling stability. However, the observed specific capacity values for Co3O4 are much lower than their theoretical values, and the specific capacity usually severely decays at high charge/discharge currents. Therefore, it is an ongoing challenge to further improve its specific capacity and rate capability.
Cobalt hydroxide (Co(OH)2) is another kind of cobalt compound that has been widely investigated for its rich redox reactions [98][111]. Compared with nickel oxide and hydroxide, cobalt hydroxide provides more electrons when a redox reaction is going on [99][100][101][112,113,114]. Furthermore, hydrotalcite-like cobalt hydroxide usually shows a positively charged Co(OH)2−x layer and an interlayered gallery with negatively charged anions (e.g., Cl−, SO42−, NO3−) [102][103][104][105][115,116,117,118]. Recently, Xu et al. [104][117] reported the preparation of α-Co(OH)2 nanoparticles by cobalt zeolitic-imidazolate frameworks (ZIF-67) hydrolyzation, and the as-prepared α-Co(OH)2 nanoparticles presented superior specific capacity of 314 C g−1 at a low current density of 1 A g−1, high rate capability (77% of capacity retention at 20 A g−1) and good cycling stability (100% of capacity retention after 20,000 cycles). In order to further improve the cycling stability, some researchers have tried to fabricate the hybrids. For instance, Gan et al. [106][119] recently reported the Co(OH)2/CoS hybrid nanostructure, which displays high rate capability (75.8% capacity retention as the current density was increased from 0.5 to 10 A g−1) and long cycling stability. Wang et al. [107][120] synthesized a Co(OH)2/nitrogen-doped porous graphene composite and realized ultrahigh specific capacity (1144 C g−1 at 2 A g−1), and long cycling stability (95.9% of capacity retention after 4000 cycles).
2.1.3. Multi-Metal Compounds
Owing to the multiple oxidation states and the synergistic effects between various metal ions, the multi-metal compounds show superior electrochemical performance for energy storage [12]. Generally, multi-metal compounds can be divided into multi-metal oxides, sulfides, and hydroxides. Multi-metal oxides, such as NiCo2O4 [108][109][110][121,122,123], ZnCo2O4 [111][112][113][114][124,125,126,127], MnMoO4 [115][116][117][128,129,130], and CoMoO4 [118][119][120][121][131,132,133,134], have been widely explored for energy conversion and storage. Among these multi-metal oxides, the NiCo2O4 nanomaterials have attracted increasing attention due to their merits of higher electrical conductivity, and higher electrochemical activity, which would offer richer redox reactions, including contributions from both Ni2+/Ni3+ and Co3+/Co4+ redox couples in the materials [108][109][110][121,122,123]. For example, Shen et al. [122][135] that reported the highly uniform NiCo2O4 hollow spheres exhibited a high specific capacity of 541 C g−1 at 1 A g−1, and excellent rate performance (74.8% of capacity retention from 1 A g−1 to 15 A g−1). In addition, it also demonstrated good cycling stability with 94.7% of capacity retention after 4000 cycles of continuous charge-discharge testing at the continuous charge-discharge testing at a current density of 5 A g−1. All these superior performances are caused by the advantageous structural features of these NiCo2O4 hollow spheres [122][135]. Ji et al. [123][136] reported a NiCo2O4 positive electrode material with an urchin-like hollow hierarchical microsphere structure, which delivered a high capacity of 424 C g−1 at 0.5 A g−1 and satisfactory rate capability (62.6% capacity retention from 0.5 A g−1 to 10 A g−1). To further enhance the electrochemical performance of NiCo2O4, many researchers have tried to design 3D porous hybrid electrode architectures by incorporating carbon materials. This hybrid architecture could solve the intrinsic poor conductivity and inevitable agglomeration of NiCo2O4 electrode materials [124][137]. For example, Li et al. [124][137] prepared the layered NiCo2O4/RGO nanocomposite and achieved an ultrahigh specific capacity of 694 C g−1 at 0.5 A g−1 and excellent cycle life with 90.2% capacity retention after 20,000 cycles at 5 A g−1. Al-Rubaye et al. [125][138] recently reported the NiCo2O4-rGO nanocomposite, which consists of NiCo2O4 hexagons wrapped in conducting rGO sheets, which exhibited a high specific capacity of 533 C g−1 at 2 A g−1 and excellent cycling stability with 98% capacity retention after 10,000 cycles. Sun et al. [126][139] reported the NiCo2O4 nanoparticle/three-dimensional porous graphene (NiCo2O4/3D-G) composite by a facile hydrothermal method combined with subsequent annealing treatment. The obtained NiCo2O4/3D-G hybrid electrode displayed a high specific capacity of 920 C g−1 at 1 A g−1. When being used as a positive electrode for HSC, the NiCo2O4/3D-G//rGO HSC device exhibited a high energy density of 73.8 W h kg−1 at a power density of 800 W kg−1 and long cycle stability with 94.3% capacity retention after 5000 cycles [126][139].
2.2. Negative Electrode Materials
The negative electrode material is also crucial in developing high-performance HSCs with high energy density and excellent rate capability. Since the different mass ratios will affect the overall capacitance of the HSC device [127][128][202,203], to balance the charges stored on the two electrodes of HSCs, the matching ratio of positive and negative electrodes should be accurately calculated. Carbon materials, such as activated carbon (AC), carbon nanotubes (CNTs), and reduced graphene oxide (rGO), are widely utilized for electrode materials in SCs due to their easy accessibility, good processing ability, large surface area/porosity, low electrical resistivity, robust surface chemical environment, physicochemical stability, and low cost [30][33]. Currently, the most commonly used electro-active materials in HSC electrodes are AC, CNTs, and rGO materials.2.2.1. Activated Carbon Materials
AC is the most commonly used negative electrode material in HSCs because of its low cost and large surface area. At present, the AC electrodes have been applied to commercial SCs with high power density. Many recent advances in AC-based HSCs have been widely reported, as summarized in Table 34. The capacitance of AC is not linearly related to its surface area and pores sizes, such that the specific capacitance of micropores is larger than that of mesopores [30][128][33,203]. Therefore, controlling the pore size distribution of AC electrodes is very important. Kierzek et al. [129][204] prepared microporous AC with a surface area in the 1900–3200 m2 g−1 range and a pore volume of 1.05 to 1.61 cm3 g−1. The capacitance values ranging from 200 to 320 F g−1 were achieved compared with the 240 F g−1 of the commercially available ACs [129][130][204,205]. AC with remarkable performance, similar to SC electrodes, has also been prepared using other methods. For instance, Zhang et al. [131][206] prepared AC by the ZnCl2 activation method, and the material exhibited a high surface area of 1935 m2 g−1 and a total pore volume of 1.02 cm3 g−1. Moreover, it showed a high specific capacitance of 374 F g−1 (1 mol L−1 H2SO4 electrolyte), excellent capacity retention, and long cycling stability. In brief, although AC has a long history of usage and production, its structural and chemical characteristics are experiencing continual evolution to meet the requirements of more demanding emergent applications [130][205].Table 34.
Summary of performances of HSCs based on AC as negative electrode.
| Device | Voltage (V) |
Energy Density (Wh kg | −1 | ) | Power Density (W kg | −1 | ) | Cycle Performance | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Zn-Ni-Al-Co oxide//AC | 0–1.5 | 72.4 | 533 | 90% (10,000 cycles) | [132][207] | ||||
| NiO/Ni3S2//AC | 0–1.6 | 52.9 | 1600 | 92.9% (5000 cycles) | [133][208] | ||||
| Ni(OH)2//AC | 0–1.3 | 35.7 | 490 | 81% (10,000 cycles) | [54][57] | ||||
| Ni(OH)2//AC | 0–1.6 | 22 | 800 | 85.7% (4000 cycles) | [64][67] | ||||
| Ni(OH)2-AB//AC | 0–1.4 | 18.7 | 1971 | 91% (5000 cycles) | [66][69] | ||||
| β-Ni(OH)2//AC | 0–1.6 | 36.2 | 100.6 | 92% (1000 cycles) | [134][200] | ||||
| rGONF/Ni(OH)2//AC | 0–1.7 | 44.1 | 467 | 77.4% (2000 cycles) | [135][209] | ||||
| NiS//AC | 0–1.8 | 31 | 900 | 100% (1000 cycles) | [136][210] | ||||
| NiS//AC | 0–1.6 | 33.4 | 800 | 87.3% (5000 cycles) | [137][211] | ||||
| Ni/Co-LDHs//AC | 0–1.6 | 165.5 | 1530 | 85% (500 cycles) | [138][212] | ||||
| ZnCo2O4//AC | 0–1.6 | 29.7 | 398.5 | 72.5% (1000 cycles) | [139][213] | ||||
| ZnCo2O4//AC | 0–1.6 | 33.98 | 800 | 93.3% (10,000 cycles) | [140][214] | ||||
| NiCo2O4/rGO//AC | 0–1.5 | 57 | 375 | 90.2% (20,000 cycles) | [124][137] | ||||
| NiCo2S4/Co9S8//AC | 0–1.5 | 33.5 | 150 | 65% (5000 cycles) | [141][144] | ||||
| CuCo2S4-HNN//AC | 0–1.6 | 44.1 | 800 | 94.1% (6000 cycles) | [142][215] | ||||
| MCS/GNF//AC | 0–1.6 | 54.26 | 1120 | 81.9% (4000 cycles) | [143][216] | ||||
| NiCo2S4//AC | 0–1.6 | 25.5 | 334 | 93.4% (1500 cycles) | [144][168] | ||||
| NiCo2S4//AC | 0–1.6 | 42.7 | 1583 | 92% (10,000 cycles) | [145][170] | ||||
| NiCo2S4 nanopetals//AC | 0–1.6 | 35.6 | 819.5 | 94.3% (5000 cycles) | [146][217] | ||||
| NiCo-LDH//AC | 0–1.6 | 69.7 | 800 | 87% (20,000 cycles) | [147][177] | ||||
| NiCo-LDH//AC | 0–1.5 | 17.5 | 10500 | 91.2% (10,000 cycles) | [148][167] | ||||
| MnCo-LDH@Ni(OH)2//AC | 0–1.5 | 47.9 | 750.7 | 90.9% (5000 cycles) | [149][182] | ||||
| NiCo-LDH//AC | 0–0.8 | 15.9 | 400 | 82.7% (20,000 cycles) | [150][183] | ||||
| NiCo2O4@NiCoAl-LDH//AC | 0–1.6 | 74.6 | 800 | 93% (2000 cycles) | [151][175] | ||||
| NiCo-LDH/graphene//AC | 0–1.7 | 68 | 594.9 | 94.2% (2500 cycles) | [152][165] | ||||
| NiFe-LDH//AC | 0–1.6 | 50.2 | 800 | 65% (2000 cycles) | [153][195] | ||||
| NiMoO4//AC | 0–1.7 | 60.9 | 850 | 85.7% (10,000 cycles) | [154][218] | ||||
| NiCo-10//AC | 0–1.6 | 51.5 | 825 | 89.5% (6000 cycles) | [155][219] | ||||
| NiSe2//AC | 0–1.6 | 44.8 | 969.7 | 87.4% (20,000 cycles) | [156][220] |
2.2.2. Carbon Nanotube Materials
CNTs have been widely studied for SCs owing to their porous structure, high surface area, good electrical conductivity, and low density [157][158][159][221,222,223]. Owing to their unique tubular structures and the high density of mesopores, they exhibit much higher specific capacitance than ACs [160][224]. Compared to multiwalled CNTs, single-walled CNTs exhibit better electrochemical properties because of their large specific surface area (~1600 m2 g−1), high aspect ratio, fast charge transport, and large accessibility of electrolyte ions [161][162][163][225,226,227]. Recently, Wang et al. [163][227] reported hierarchically porous CNTs by a simple carbonization treatment, which displayed a high specific surface area of 1419 m2 g−1 and hierarchical micro-/meso-/macroporosity. This unique porous architecture delivered an ultrahigh specific capacitance of 286 F g−1 at 0.1 A g−1, and excellent rate capability (~71% capacity retention from 0.1 to 50 A g−1) [163][227]. To increase the energy and power density of devices, other strategies have also been -employed, such as atomic doping and combining CNTs with other materials (e.g., metal oxides, ACs, and graphehe) [164][165][166][167][228,229,230,231]. For example, Kim et al. [166][230] recently reported a polyimide/MWCNT composite electrode with a high specific capacitance of 333.4 F g−1 at 1 A g−1. Jin et al. [167][231] reported a polyaniline/carbon nanotubes/graphene/polyester hybrid electrode with a high areal capacitance of 791 mF cm−2 at a current density of 1.5 mA cm−2. Although various types of research have been carried out on CNTs for HSCs, most of the reported electrodes are often in powdered form or have a disordered texture with poor interconnectivity among micro-/mesoporous structures, which leads to a low specific capacitance and high internal resistance, thus resulting in a much lower energy and power density for devices [168][169][170][232,233,234]. Therefore, it is still a great challenge to further improve its performance.2.2.3. Reduced Graphene Oxide Materials and Their Hybrids
Another promising negative electrode material for HSCs is graphene. Graphene, a two-dimensional carbon sheet with monoatomic layer thickness, has been widely explored as an ideal electrode material for SCs due to its unique properties, including its high theoretical surface area (2630 m2 g−1) and high in-plane electrical conductivity [171][172][235,236]. It has brought a sensational revolution in the field of energy storage and conversion. To date, various routes have been developed to fabricate graphene sheets, such as blade-coating, spray-coating, layer-by-layer assembly, interfacial self-assembly, and filtration assembly [173][174][175][176][177][178][237,238,239,240,241,242]. In principle, a supercapacitor based on graphene is capable of achieving a theoretical electrochemical double layer capacitance as high as 550 F g−1 [179][180][243,244]. However, the practical performance of graphene is far below the ideal one due to various reasons. One of the main reasons is that the 2D layered graphene sheets can easily restack to form dense lamellar microstructures, which greatly decreases the specific surface area of the original graphene sheets, causes inferior ion transport capabilities, and renders a substantial number of active sites inaccessible to reactants [181][182][183][184][245,246,247,248]. Therefore, a number of strategies have been developed to prevent aggregation of graphene sheets so as to increase surface area and promote the transport of electrolyte ions, including fabricating 3D porous nanostructures [185][186][249,250], nitrogen doping [187][188][189][251,252,253], and surface modification using molecular modifiers [190][191][254,255]. Modifying the surface structure of electrode materials could improve their compatibility with electrolytes, enrich redox sites, and enhance the surface conductivity, leading to good electrochemical performance [30][33]. Recently, oxygen- and nitrogen-containing groups have been well studied to modify the graphene surface. For example, Song et al. [190][254] recently reported different functionalized graphene networks by using amine molecules and a facile two-step hydrothermal method. The as-fabricated graphene composite exhibited an improved capacitance and fast ionic diffusion features in aqueous and organic electrolytes, with less than 10% capacitance decay during 10,000 charge/discharge cycles [190][254]. Li et al. [192][257] reported chemical compounds of GO and amine molecules as spacers by one-step hydrothermal reactions. The as-prepared graphene composite electrode exhibited excellent performance with a high specific capacitance of 597 F g−1 [191][255]. In conclusion, heteroatoms in doped graphene materials play a key role in electron transfer and energy conversion processes. The incorporation of nitrogen or molecular modifiers can provide the work electrodes with high-density active sites to enhance the capacitance performance. Moreover, it can also reduce the agglomeration level of graphene and create few-layer graphene sheets with interconnected open pores, which provide an effective pathway for charge transport.3. Design Structures of Hybrid Supercapacitors
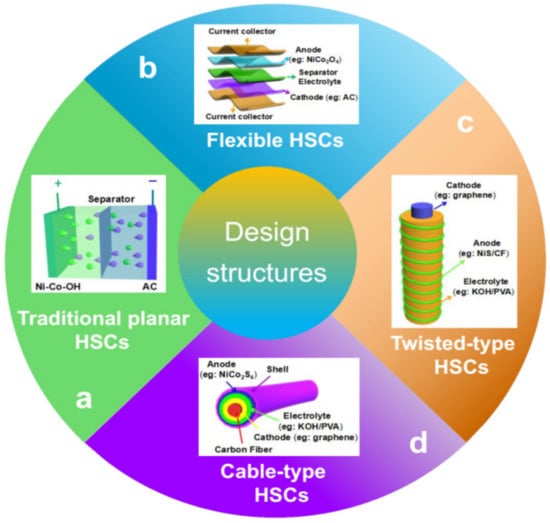
A HSC device usually contains positive and negative electrodes, an electrolyte, a separator (to prevent short circuits between electrodes), and current collectors. Besides the electrodes, electrolytes also play an important role in HSC performance. The electrolytes of HSCs could be organic (LiPF6, LiBF4, LiClO4, NaClO4, NaPF6, etc.), ionic liquid (BMIMBF4), gel-polymer (PVA-H3PO4, PVA-LiCl, etc.), or aqueous of acidic (H2SO4, CH3SO3H), alkaline (KOH, NaOH), and neutral (Na2SO4, Li2SO4) [13]. Aqueous electrolytes usually have the advantages of high ionic conductivity, low cost, non-flammability, safety, and convenient assembly in air [193][261]. But its potential window is limited to 1.2 V, which is far lower than that of organic electrolytes (3.5–4 V). A high-potential window is a large merit for organic electrolytes, which could significantly contribute to high energy density. However, it is less conductive, expensive, usually flammable, and more toxic [13][193][13,261]. Ionic liquids as nonvolatile, highly stable electrolytes are considered as the most promising electrolytes compared to organic ones for HSC applications [13]. The gel-polymer electrolyte is usually used for designing and fabricating flexible/stretchable or even smart HSCs due to its merits of avoiding electrolyte leakage or without using an additional separator [194][195][262,263]. Four main types of HSCs are summarized in Figure 3.
Figure 3. The hybrid supercapacitors with four representative structure types, namely, (a) traditional planar HSCs, (b) flexible HSCs, (c) twisted-type HSCs, and (d) cable-type HSCs.
