Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Fanny Huang and Version 1 by Paweł Koperski.
Invertebrates are excluded from ethical consideration in the procedures of environmental protection, which results in the killing of many more individuals during sampling than necessary. Biomonitoring is used as a routine method for environmental protection that results in the cruel death of even millions of aquatic animals annually.
- Invertebrates
- freshwater environment
- biomonitoring
1. Introduction
“It is clear that we have direct ethical obligations to sentient animals; but it is not at all clear that we have direct ethical obligations to entities such as species, or to biological diversity. The burden of proof should thus be on conservationists to show how killing the first to preserve the second can possibly be acceptable from an ethical point of view” [1]. Biological monitoring (biomonitoring), in the broadest sense, can be defined as activities aimed at assessing the condition of the environment, performed according to an agreed methodology and using bioindicators. It consists of the assessment of different parameters and detecting ongoing changes in ecosystems and components of biological diversity, including types of natural habitats, populations and species, as well as to assess the effectiveness of the nature protection methods used. In this approach, biological monitoring aims not only to assess the state of the natural environment, organisms and ecosystems, but also other environmental parameters, such as the degree of habitat transformation, and soil, air and water pollution. Some methods are based on the presence or relative abundance of specific taxonomic groups in the environment. The assumption of some of the biomonitoring methods, especially those aimed at assessing the presence of xenobiotics in the environment and their effects, depends on the collection of whole organisms, body fragments or various physiological secretions for analysis.
The Convention on Biological Diversity [2] encourages states that are parties to it to monitor the elements of biological diversity, with particular emphasis on its most endangered components and those representing the greatest potential value for sustainable use. This should include, in particular, monitoring the effects of processes and activities that have or may have a significant negative impact on the conservation and sustainable use of biological diversity. According to the convention, environmental monitoring should cover all levels of biodiversity from ecosystems, through to species level, to genetic diversity.
Since the 1980s, this type of activity has been most intensively carried out in freshwater environments. In European Union countries, in accordance with the requirements of the Water Framework Directive of the European Parliament (WFD) [3], the basis for this type of assessment is the analysis of the composition of the so-called “biological elements”. This term means groups of organisms with a confirmed high indicative value. Indicator organisms can be used for biological monitoring purposes at various organizational levels, from the sub-organismal level (e.g., genes, cells, tissues) through the organismal level, to populations, communities or even a whole ecosystem level [4]. Among the five biological elements used to assess the ecological status of European freshwater environments: fish, macrophytes, phytoplankton, periphyton algae and macroinvertebrates, the latter is the most often and most commonly used. Macroinvertebrates remain on the sieve with a mesh size of 0.2–0.5 mm, i.e., in practice have a body length exceeding 1 mm. It is estimated that about two-thirds of the methods for assessing the quality of flowing waters are based on benthic macroinvertebrates.
About 15,000 species of Metazoa occur in European waters, of which about 10,500 can be termed macroinvertebrates. The vast majority of those used in monitoring programs are insects, in particular their larval forms. However, the less numerous groups include water-mites (Acari: Arachnida), snails and mussels (Mollusca), leeches and oligochaete worms (Clitellata), malacostracan and other groups of so-called Crustacea. Insects contain the taxa with the highest indicative value e.g., the EPT group (Ephemeroptera, Plecoptera, Trichoptera larvae). Many species of freshwater invertebrates in Europe are endangered [5] and are particularly sensitive to habitat change as flow alterations, habitat fragmentation, long-lasting drought and pollution are their main threats [6]. The recently intensively studied global decrease in the number and biomass of insects, including ecologically specialized rare species of aquatic insects, is undoubtedly related to the degradation of freshwater environments [7].
A significant part of the waters located in areas of intensive human impact are degraded, and their functional, hydrological and chemical parameters as well as the taxonomic composition of the organisms inhabiting them significantly differ from the pristine conditions. Routine assessment of the ecological status of these environments is carried out at designated sites by official institutions appointed by the authorities to collect data on ecosystem degradation. Freshwater environments—lakes, ponds, wetlands, streams and rivers—are some of the most threatened habitat types on Earth. They contain less than three percent of the volume of water stored on Earth, but about ten percent of animal species live in them. Typically, a large part of terrestrial biodiversity is concentrated near freshwater [8]. Declining numbers of aquatic invertebrates have negative effects on ecosystems because the populations of many species are essential for their function. These organisms filter a huge amount of edible particles suspended in water, control prey populations as predators, constitute the food base of fish species and consume periphytic algae and dead organic matter, which accelerates the biomass turnover. The activity of certain groups reduces the effects of eutrophication and intensifies the self-purification processes in watercourses. Huge swarms of winged mayflies, true-flies and caddisflies after their emergence constitute an irreplaceable food base for many terrestrial vertebrates [9]. As significant reductions in their abundance may cause disturbances in the functioning of the ecosystem, it means that excessive mortality can be regarded as a kind of environmental degradation.
2. Use of Freshwater Invertebrates in Biomonitoring
A typical method of assessing the ecological status of a freshwater environment, in accordance with the requirements of the WFD, is based on the following points: (i) selection of the field site, as assigned to the appropriate abiotic type; (ii) quantitative sampling of invertebrates from the appropriate bottom area, using specialized equipment; (iii) preserving the samples using a suitable substance; (iv) random sampling in the laboratory from the preserved organisms of sub-samples of the total sample until the appropriate number of specimens are obtained, e.g., the minimum for proper evaluation; (v) identification of animals selected in sub-samples to the required level (typically the level of family); (vi) calculation of the value of the multimetric biotic index on the basis of taxonomic composition and richness of the invertebrate fauna; (vii) determination of the class of the ecological status (on a five-point scale) on the basis of the final index score depending on the habitat type [11][10]. A detailed description of the protocol is available online [12][11].
It should be noted that new versions of protocols developed for the assessment of freshwater environments increasingly contain propositions intended to reduce the mortality of at least some invertebrates, without decreasing the quality of the assessment. There, you can find suggestions to return to the environment as many individuals as possible whose identification is possible already in the field, e.g., mussels from the Unionidae family, crayfish, as well as to review samples in the field for the presence of protected species and if they occur, their presence should be recorded in field protocol, and the animals removed from the sample and left in the environment [12][11]. In practice, during field analysis, such a procedure may be only partially effective, e.g., due to the inability to see the younger (smaller) developmental stages of large animals and the inability to distinguish in situ individuals of protected and unprotected species (e.g., dragonflies or beetles).
The methods of biological assessments of freshwater environments differ in both the sampling methods and the biological variables used [13][12]. Most published studies and reports for different reasons do not mention the total number of animals killed (preserved) but only the number of animals used for analysis. Therefore, it is very difficult to directly compare the levels of invertebrate mortality during routine biological monitoring carried out by different methods. Important factors affecting such mortality rates are: sampling area, methods, intensity, mesh size, sample replications and handling methods of trapped animals (live sorting, preservation of the whole sample, non-lethal collection or identification of selected taxa). Some procedures which are applied in the laboratory post-preservation (i.e., the use of magnification during sorting, sub-sampling and the level of identification) appear to have no effect on mortality, because they only reduce the number of animals used for analysis after preservation, and thus killing all; regardless of the method, it is never an easy “humanitarian” death [11][10].The effects of using the described sampling methodology in the routine monitoring of flowing waters in Poland between 2011 and 2018 were analyzed in detail [10,11][10][13]—this will be used to illustrate general patterns. The methods of biomonitoring in lakes, dam reservoirs and coastal brackish waters used in Poland are so similar that the effects caused by them are probably similar [12][11]. It can be assumed that the conclusions from a detailed analysis of the effects of sampling in routine monitoring in other countries using methods based on similar assumptions and defined by the requirements of the WFD would be similar, e.g., [14,15][14][15]. The most important direct effects of the described procedure of preserving entire bulk samples and the use of subsample analyses in the monitoring of flowing waters are:
-
The number of aquatic invertebrates killed (preserved) during sampling is sometimes very high, which significantly exceeds the minimum number necessary for a correct assessment of the state of the environment;
-
A significant number of the animals killed during conservation are not used for analysis, which receives much less abundant sub-samples;
-
There are significant variations in the numbers of animals killed during maintenance, involving the taking of samples for analysis by staff from different laboratories, following the same protocols and often in very similar types of environments.
The number of animals killed during the sampling procedure exceeds, on average, 12 times the number necessary for proper analysis (the described method requires placing at least 350 identified individuals in the database intended for the calculation of the index). In sixty-one percent of the samples, at least five times as many animals were killed than the number sufficient for analysis, in some cases reaching even over 200–500 times more. Moreover, it can be estimated that these numbers are actually even higher due to the fact that for taxa occurring in high densities, the procedure allowed to record only the first 100 individuals sampled, but typically it is not noted in the archived data [12][11].
As a result of the described subsampling procedure, 80.4% of the animals killed as a result of conservation were not used for the assessment of the environment and died, so to speak. Among the animals of which this type of misuse applied, insects (63.4% of individuals) and Malacostraca (17.3%) predominate. Identifying animals only at the family level means that the collected data cannot be used for scientific purposes (e.g., biodiversity analysis) and it also makes it impossible to reliably assess the impact of the procedure on the populations of rare and protected species. For example, over 10,000 individuals of the mussel family Unionidae were killed, among which there are rare, vanishing and protected species, of which about 8300 individuals, despite being killed, were not included in the analysis. In the case of the Heptageniidae family (Ephemeroptera, mayflies), which also includes rare, endangered and protected species, these numbers are 125,455 (killed specimens), 24,577 (number enough for analysis) and 100,878 (not used for analysis), respectively. In total, there were 2,817,777 specimens which belonged to families, including species covered by various forms of legal protection.
It has been estimated that between 2012 and 2019, at least 12.7 million invertebrates (more than 8.6 million aquatic insects) fell victim to biomonitoring in Poland’s watercourses (Figure 1), and according to very rough estimates, on a European scale it was about 18 million animals per year [10,11][10][13]. It is very difficult to assess what effects such mortality has on aquatic invertebrate populations and the functioning of ecosystems. This type of impact can only be expected locally in specific environments or directed in isolated populations living in low densities. The differences in the average numbers of invertebrates killed between laboratories performing routine biomonitoring can be extreme (up to 35 times). Importantly, this does not usually stem from the effect of differences in animal densities, resulting from the ecological and geological differences between environments. The average number of invertebrates collected by the staff of different laboratories from one square meter patches at the bottom of watercourses of the same abiotic type (and thus very similar in terms of hydrological, geological and ecological parameters) differed even up to thirty-two times. In the analysis, much attention was paid to the hypothetical reasons for such large differences in mortality values during sampling [10][13]. The overall variance associated with the total number of insects killed during sampling is explained mainly by: (i) the specificity of the laboratory and (ii) the specificity of the abiotic type; the first factor is much more important than the second one. The remaining analyzed factors are of very little importance. This clearly indicates that it is the “human factor” that is difficult to define—the level of empathy in dealing with invertebrates, which varies greatly among individual people taking samples, is the most important in this case. More important than ecological factors, freedom in the use of the sampling equipment allowed by the procedure and even the aforementioned lack of mechanisms limit the maximum sample abundance.
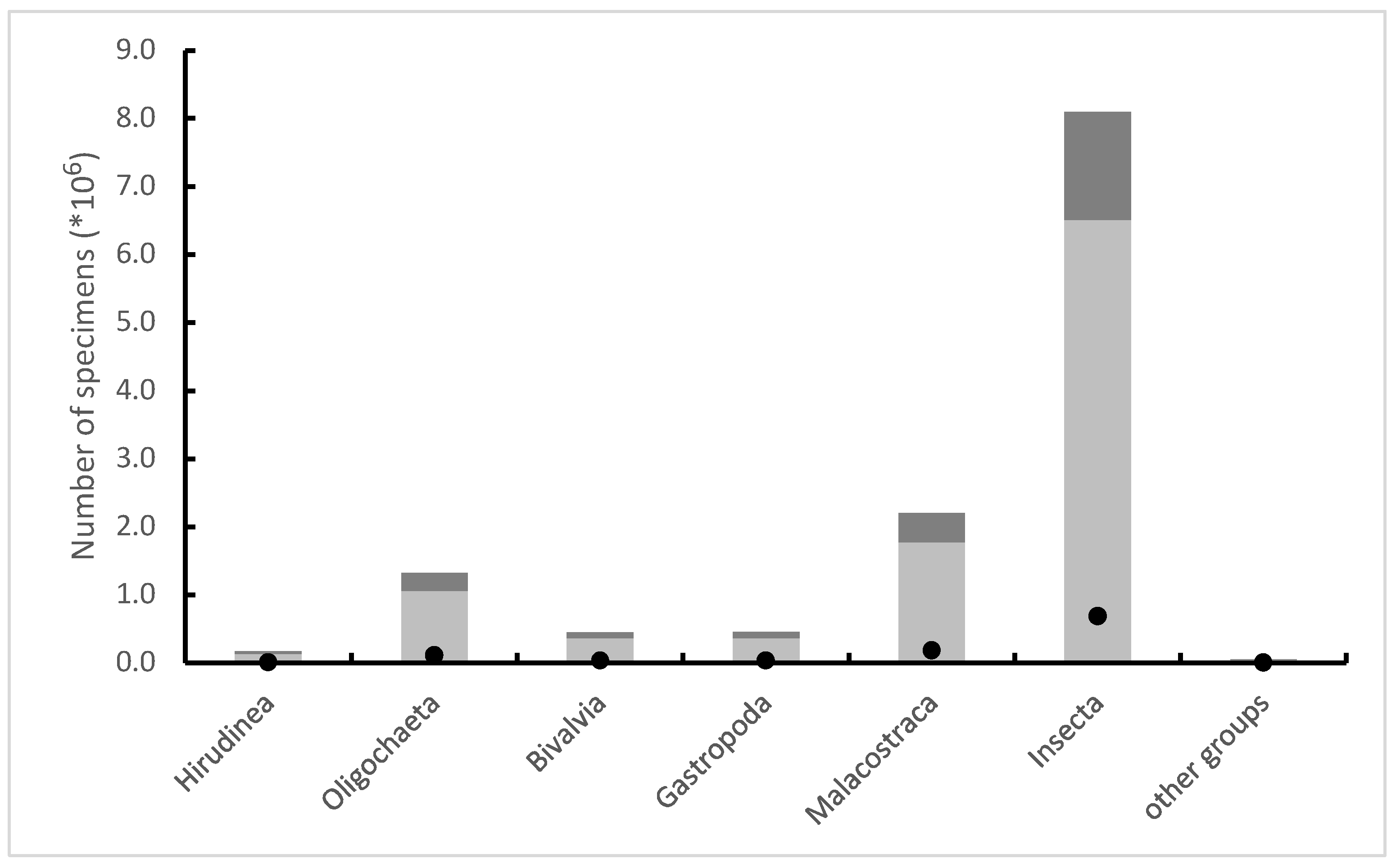
Figure 1. Estimated number of animals from the most important taxonomic groups that were killed during sampling for the purposes of biomonitoring in Polish watercourses in 2012–2019, with the number of specimens used for the analysis (dark grey) and the number of individuals necessary for the correct analysis (black dots) shown.
References
- Rawles, K. Biological Diversity and Conservation Policy. In Philosophy and Biodiversity; Oksanen, M., Pietarinen, J., Eds.; Cambridge University Press: Cambridge, UK, 2004.
- Convention on Biological Diversity. 1992. Available online: https://www.cbd.int/convention/text/ (accessed on 13 May 2016).
- Water Framework Directive. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000; Establishing a Framework for Community Action in the Field of Water Policy. 2000. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32000L0060 (accessed on 20 November 2014).
- Bonada, N.; Prat, N.; Resh, V.; Statzner, B. Developments in aquatic insect biomonitoring a comparative analysis of recent approaches. Ann. Rev. Entomol. 2006, 51, 495–523.
- Czachorowski, S.; Buczyński, P. Zagrożenia i ochrona owadów wodnych w Polsce. Wiad. Entomol. 1999, 18 (Suppl. S2), 95–120, (In Polish, English Summary).
- Sánchez-Bayo, F.; Wyckhuys, K.A. Worldwide decline of the entomofauna: A review of its drivers. Biol. Cons. 2019, 232, 8–27.
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809.
- Cantonati, M.; Poikane, S.; Pringle, C.M.; Stevens, L.E.; Turak, E.; Heino, J.; Richardson, J.S.; Bolpagni, R.; Borrini, A.; Cid, N. Characteristics, Main Impacts, and Stewardship of Natural and Artificial Freshwater Environments: Consequences for Biodiversity Conservation. Water 2020, 12, 260.
- Suter, G.W.; Cormier, S.M. Why care about aquatic insects: Uses, benefits, and services. Integr. Environ. Assess. Manag. 2015, 11, 188–194.
- Koperski, P. Freshwater Invertebrates—Neglected Victims of Biological Monitoring: An Ethical View. Ethics Environ. 2022, 27, 29–57.
- Kolada, A. (Ed.) Podręcznik do Monitoringu Elementów Biologicznych i Klasyfikacji Stanu Ekologicznego Wód Powierzchniowych. Aktualizacja Metod; Biblioteka Monitoringu Środowiska: Warsaw, Poland, 2020; (In Polish, English Version).
- Friberg, N.; Sandin, L.; Furse, M.T.; Larsen, S.E.; Clarke, R.T.; Haase, P. Comparison of Macroinvertebrate Sampling Methods in Europe. In The Ecological Status of European Rivers: Evaluation and Intercalibration of Assessment Methods; Furse, M.T., Hering, D., Brabec, K., Buffagni, A., Sandin, L., Verdonschot, P.F.M., Eds.; Springer: Dordrecht, The Netherlands, 2006.
- Koperski, P. Local variability, human factor or vague procedure? Searching for the reasons of excessive mortality in free living aquatic insects, resulting from biological monitoring. J. Insect Conserv. 2023, 27, 589–599. Available online: https://link.springer.com/article/10.1007/s10841-023-00482-y (accessed on 2 June 2023).
- Escribano, N.; Oscoz, J.; Galicia, D.; Cancellario, T.; Durán, C.; Navarro, P.; Ariño, A.H. Freshwater macroinvertebrate samples from a water quality monitoring network in the Iberian Peninsula. Sci. Data 2018, 5, 180108.
- AQEM Consortium. Manual for the Application of the AQEM System. In A Comprehensive Method to Assess European Streams Using Benthic Macroinvertebrates, Developed for the Purpose of the Water Framework Directive; Version 1.0. February. 2002. Available online: https://www.eugris.info/displayproject.asp?Projectid=4422 (accessed on 28 September 2006).
More
