Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Gianluigi Giannelli and Version 2 by Lindsay Dong.
The use of beneficial microorganisms inhabiting the rhizosphere (PGPR) is a promising tool to alleviate salt stress in various crops and represents a strategy to increase agricultural productivity in salt soils. Increasing evidence underlines that PGPR affect plant physiological, biochemical, and molecular responses to salt stress. The mechanisms behind these phenomena include osmotic adjustment, modulation of the plant antioxidant system, ion homeostasis, modulation of the phytohormonal balance, increase in nutrient uptake, and the formation of biofilms
- plant-growth-promoting rhizobacteria (PGPR)
- salt stress
- plant salt-responsive genes
- PGPR plant gene modulation
1. Rhizosphere Microbial Community
Since their earliest evolution, land plants have been associated with a complex microbial community that includes beneficial microorganisms. The presence of beneficial microorganisms helped early land plants to respond to the environment and face challenges such as access to nutrients, new and/or stressful conditions, and pathogens [1][2][12,13].
In soil, there is a gradient of intimacy between plant roots and microorganisms, and the plant’s influence on the microbial community is greater near the root surface. A volume of soil specially influenced by plant roots or associated with the roots and plant-produced material is defined as a rhizosphere [3][14], a term originally coined by Hiltner [4][15] to describe the microorganisms of soil around and inside roots. Microorganisms living on the surface of roots are now said to inhabit the rhizosphere and the rhizoplane, and those living inside the roots are called endophytes [5][6][16,17].
Among the microbial communities colonizing the different plant regions (i.e., flowers, fruits, stems, leaves, and roots), the one associated with the roots (rhizobiome) is undoubtedly the most populated and most sophisticated of all microbial communities associated with high plants [1][12]. The rhizosphere effect is caused by the enormous influence exerted by the plant root exudates, a phenomenon called rhizodeposition, including a range of organic acids, amino acids, sugars, nucleosides, vitamins, and other small molecules that act as strong chemo-attractants of the soil microbiota [7][18]. Rhizodeposition also refers to the release into the rhizosphere of a group of specialized cells, called root cap boundary cells. This cell type is considered crucial for its effect on the rhizosphere as it usually survives after it has decayed from the roots [8][9][19,20] In particular, the passive flux of low-molecular-weight metabolites such as sugars, amino acids, and organic acids through root exudation is mostly located at the root tip, where the lack of cell differentiation favors the diffusion of metabolites to the soil [10][21].
The root tip senses changes in the concentration of exuded metabolites and translates them into signals to modify root growth. Through root exudate flux, plants can locally enhance concentrations of many common metabolites, which can serve as sensors and integrators of the plant nutritional status and of the nutrient availability for the rhizosphere microbial community [11][12][22,23]. Plant-associated microorganisms also constitute a strong sink for plant carbon, thereby increasing concentration gradients of metabolites and affecting root exudation. Indeed, it was found that when microorganisms were present in plant growth solution, exudation was enhanced compared to axenic conditions [13][24]. Rhizodeposits account for approximately 11% of the net carbon fixed by photosynthesis, and 10–16% of the total nitrogen in plants, but these values vary greatly depending on plant species and age [14][25]. The net sequestration of organic carbon and nitrogen by roots stimulates the multiplication of soil microbes near root tissues because (i) most known soil bacteria are organotrophic (i.e., they obtain growth energy from organic substrates) and (ii) in most soils, the access and availability of organic compounds are limited.
The microbial population colonizing the rhizosphere includes bacteria, which are the most abundant, as well as fungi, protozoa, and algae, and its composition is markedly different from that of its surrounding soil [15][16][26,27]. Regarding bacteria, Weller and Thomashow [17][28] proved that their presence in the rhizosphere is generally 10 to 100 times greater than in bulk soil. All plant–microbe interactions can have mutualistic or detrimental effects on the host during the microbes’ attempts to obtain nutrients and environmental protection, regardless of the physical interaction [18][29]. For this reason, plants need a selection mechanism that favors rhizosphere colonization by beneficial and non-pathogenic microorganisms.
The literature available suggests a two-step selection process that gradually distinguishes the root microbiota from the surrounding soil biomes and determines differences in the dimension and composition of the microbial community. In the first step, it is suggested that rhizodeposition and the recognition of the host cell wall promote organotrophic bacteria growth, thereby initiating the first community shift of the soil biome. In the second step, the microbial subpopulation undergoes a second selection process near and within the roots, based on host genotype-dependent selection, defining the profiles of the microbial communities that prosper in the rhizoplane and within the roots [8][19][19,30].
2. Plant-Growth-Promoting Rhizobacteria (PGPR)
In 1978, Kloepper and Schroth [20][32] introduced the term “rhizobacteria” to indicate the soil bacterial community capable of competitively colonizing plant roots and stimulating growth, thereby reducing the incidence of plant disease. They were then renamed plant-growth-promoting rhizobacteria in 1981 [21][33]. PGPR represent about 2–5% of the total rhizosphere bacteria [22][34]. According to Nakkeeran et al. [23][35], an ideal PGPR should possess high rhizosphere competence, improve plant growth capabilities, have a wide range of action, be safe in relation to the environment, compatible with other rhizobacteria, and tolerant to heat, UV radiation, and oxidants. Based on the interaction with plants and their degree of association, PGPR can be classified as either intracellular PGPR (iPGPR or symbiotic bacteria), which live inside the plants and exchange metabolites with them directly, or free-living rhizobacteria or extracellular PGPR (ePGPR), which live outside plant cells [5][24][16,36]. iPGPR include a wide range of soil bacterial genera that are usually located inside the specialized nodular structures of root cells such as Allorhizobium, Azorhizobium, Bradyrhizobium, Mesorhizobium, and Rhizobium of the family Rhizobiaceae [25][37]. ePGPR, instead, are present in the rhizosphere, in the rhizoplane, or in the spaces between the root cortex cells, and include genera such as Agrobacterium, Arthrobacter, Azotobacter, Azospirillum, Bacillus, Burkholderia, Caulobacter, Chromobacterium, Erwinia, Flavobacterium, Micrococcous, Pseudomonas, and Serratia [5][16]. The beneficial action of PGPR is exerted through a series of processes that result in three macro effects on the host plant: (i) increased nutrient assimilation; (ii) increased resistance to phytopathogen infections (viruses, fungi, bacteria, nematodes, insects, etc.), also referred to as biotic stresses; and (iii) increased tolerance to abiotic stresses (heavy metal in soils, drought, nutrient deficiency, salinity, temperature). This leads to improved plant performance and, consequently increased growth. The diverse mechanisms underlying these phenomena include (i) N2 supply through biological nitrogen fixation; (ii) the production of siderophores; (iii) the solubilization and mineralization of nutrients; (iv) the synthesis of ACC (1-aminocyclopropane-1-carboxylate) deaminase; (v) the production and modulation of phytohormones (e.g., indole 3-acetic acid (IAA), cytokinins (CK), abscisic acid (ABA), gibberellic acid (GA)); (vi) the control of plant pathogens through various mechanisms such as the secretion of enzymes able to hydrolase the fungal cell wall or compete for nutrients, the induction of systemic resistance (ISR), and the production of siderophores and antibiotics; (vii) the bio-remediation of heavy-metal-contaminated soils; and viii) an improvement in abiotic stress resistance [26][27][28][29][30][38,39,40,41,42].34. Salt Stress Affects Plant Growth
3.1. Soil Salinization
4.1. Soil Salinization
Soil salinity is a major abiotic stress in agricultural crop productivity worldwide. The causes of soil salinization can be grouped into primary, due to natural sources, and secondary, which are largely anthropogenic and exacerbate the problem of salinization [31][32][9,43]. A primary source is saline parent material that releases soil mineral constituents during the chemical erosion of rock or sediment minerals, which react with air and water to produce soluble salts that are then transported away from their source of origin through watercourses. Another factor determining the development of saline soils is salty groundwater that rises through the soil profile via capillarity and releases salts, which then remain in the soil due to water evaporation. Coastal areas are particularly prone to salinization, since winds and sea spray along the coast transport salts from the sea inland in sufficient quantities to cause salinization of these areas. Sea spray can have an impact up to 80 km inland or even beyond [31][9]. The main secondary cause is irrigation. Irrigation can cause salinity when salty water is used or when it causes the inadequate leaching of salts into the soil. Irrigation water can also recharge underground wells and cause them to rise, gradually introducing the salts contained in groundwater into the soil. The use of fertilized water can also contribute to the introduction of salts, which accumulate over time and increase with repeated application. Waste and wastewater are also secondary contributors to soil salinity, and so is variation in land use or land cover. Soil is generally considered saline when the electrical conductivity (EC) of the saturation extract in the root zone exceeds 40 mM at 25 °C, with 15% of unbound Na+ ions [33][11]. Among the different ions that can cause salinization, sodium is the most soluble and widespread, and it is considered the most deleterious. In fact, unlike other ions, such as Ca2+, Mg2+, or K+, which plants can exploit for normal physiological and biochemical processes, Na+ is useless and extremely toxic to glycophytes [34][44]. Soils provide ecosystem services that are essential to human life and biodiversity. The salinization of soils has a serious impact on these services, as it leads to a number of consequences including (i) a reduction in agricultural productivity, water quality, soil biodiversity, and an increase in soil erosion; (ii) a decreased ability to act against contaminants as buffers and filters; (iii) a degraded soil structure; (iv) decreased functions of ecological systems such as the hydrological and nutrient cycles; (v) an increased concentration of ions that are toxic to plants; (vi) a reduced ability of crops to take up water; and (vii) reduced soil fertility and availability of micronutrients [35][6].3.2. Plant Response to Salt Stress
4.2. Plant Response to Salt Stress
Salinity stress involves changes in various plant physiological and metabolic processes, depending on the severity and duration of the stress, and ultimately inhibits plant growth. High salinity affects plants in several ways: water stress, ion toxicity, nutritional disorders, oxidative stress, the alteration of metabolic processes, membrane disorganization, a reduction in cell division and expansion, and genotoxicity [36][45]. It is not surprising that plants have evolved mechanisms to regulate NaCl accumulation, since NaCl is the most soluble and widespread salt. Analyses of the different growth responses under salinity conditions reveal that plants differ considerably in their tolerance [37][46]. Barley is the most tolerant crop among cereals, while rice is the most sensitive. Similarly, soft wheat (Triticum aestivum) is moderately tolerant, whereas durum wheat (Triticum turgidum ssp. Durum) is more sensitive to salt stress. Tall grass (Thinopyrum ponticum, syn. Agropyron elongatum) is a halophytic relative of wheat and is one of the most tolerant plants among monocotyledons. An elevated salt concentration reduces shoot growth and affects plants in two main ways: it reduces the roots’ osmotic potential, thereby complicating water uptake (osmotic effect), and inside plants, it results in ion toxicity (ionic effect) [38][48]. A two-phase model has been proposed to describe the effects of salt stress. The osmotic phase begins immediately after the exposure of the roots to a threshold level of salinity, which is usually 40 mM NaCl or less for plants such as Arabidopsis. During this phase, the immediate response is stomatal closure, which also helps to reduce ion flux to the shoot; the rate of shoot growth declines, and new leaves emerge more slowly [36][37][45,46]. However, this is an unsustainable solution and is bound to be discontinued in a short time because of the difference in water potential between the atmosphere and the leaf cells, but especially due to the need to fix carbon. Usually, osmotic stress has an immediate and greater effect on growth rate than ionic stress, except for cases with a high salinity concentration or in sensitive species that do not have a strong ability to control Na+ transport. For most plant species, Na+ toxicity is greater than Cl− toxicity; for this reason, almost all studies have focused on the mechanisms involved in Na+ control rather than Cl− [37][46]. The main physiological, molecular, and biochemical mechanisms for survival under high salt concentration include (i) ion homeostasis and compartmentalization, (ii) ion transport and uptake, (iii) the biosynthesis of osmoprotectants and compatible solutes, (iv) the activation of the antioxidant enzyme and synthesis of antioxidant compounds, and (v) the modulation of hormones [39][40][49,50].3.3. PGPR Growth and Activity in Salt Stress Conditions
4.3. PGPR Growth and Activity in Salt Stress Conditions
Most of the studies aiming to dissect PGPR activities under salt conditions are conducted in vitro, yet these observations are not directly correlated with soil in nature. Increasing NaCl concentration from 0 to 1.2 M NaCl progressively reduces the amount of ACC deaminase activity, IAA, P-solubilization, and EPS in Bacillus pumilus JPV11 [41][53]. A 38% reduction in IAA production was observed in B. megaterium when subjected to 5% salinity, while it increases in P. fluorescens cultures. When growing in 2% NaCl, V. paradoxus reduces the production of siderophores. No changes were observed in siderophore production for the other two strains, while a slight reduction was observed when increasing the salt concentration. There was no significant change in the ACC-deaminase activity of P. fluorescens and B. megaterium with salt concentrations, but there was a reduction in V. paradoxus [42][54].3.4. Contribution of PGPR to Plant Salt Tolerance
4.4. Contribution of PGPR to Plant Salt Tolerance
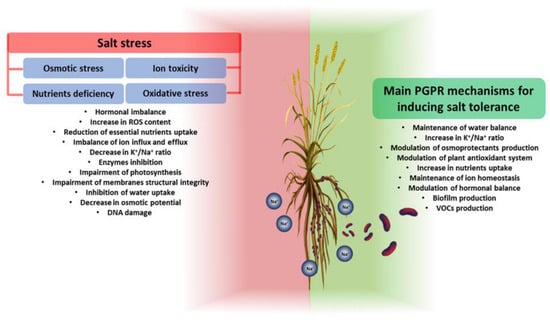
PGPR exhibit beneficial traits that allow them to mitigate the toxic effects caused by high salt concentrations. PGPR can improve plant growth in a salt environment in two main ways: (i) activating or modulating the response systems of plants during exposure to salt; and (ii) synthesizing anti-stress molecules [43][59]. Mechanisms to improve the growth and resistance of plants exposed to salinity include (i) the improvement of nutrient uptake (e.g., N2 fixation, release of bound P and K+ from the soil, chelating iron) and maintenance of the water balance; (ii) an influence on ion homeostasis; (iii) the induction of the selective absorption of K+ and exclusion of Na+ to maintain a high K+/Na+ ratio; (iv) the formation of biofilm to reduce Na+ toxicity; (v) changes in root architecture; (vi) the modulation of the antioxidant system; (vii) the modulation of osmotic substances; (viii) the modulation of plant hormonal levels; and (ix) the modulation of the expression of salt-responsive genes [44][60]. GPR are classified according to their mechanisms, but in the context of salt stress the analysis of their influence on the plant’s response has shown that their promoting activity is never due to a single mechanism. The roles of PGPR in enhancing plant salt tolerance by modulating different genes and plant cellular functions can be seen in Figure 1.
Figure 1. General overview of salt stress effects on plants (on the left) and PGPR mechanisms involved in plant salt-stress tolerance (on the right). Salt stress negatively affects all plant growth and development processes. Through different mechanisms, PGPR can increase plant tolerance to salt stress.
34.4.1. Osmotic Adjustment
A decrease in osmotic pressure induces water loss and represents one of the problems plants face when growing in saline soils, because changes in osmotic pressure must be compensated for to maintain cell volume and turgor [45][93]. In order to maintain osmotic balance both inside and outside the cell, plants increase the synthesis of low-molecular-weight compatible solutes in the cytosol, also referred to as osmolytes. The most common osmolytes include proline, glycine betaine, soluble sugars, and ectoine [46][47][94,95]. Osmolytes are not charged, polar, and soluble, and do not interfere with cell metabolism even at high concentrations [39][49]. Osmotic adjustment is an energy-intensive process; in fact, it results in a reduction in growth to direct energy to osmolyte synthesis. On the other hand, it is a necessary measure to alleviate the effects of salt stress [48][49][96,97]. Proline also acts as a scavenger for stress-induced ROS; buffers the cell redox potential; and stabilizes proteins, enzymes, membrane structures, and the electron transport system complex II [50][51][52][98,99,100]. Moreover, proline is considered a sensitive physiological marker of salt stress.
In plants, ABA signaling and MAP kinases are involved in the production and accumulation of osmolytes [53][101]. The rapid activation of MAPKs such as MAPK3, 4, 6 was frequently observed in response to salt stress [54][102]. For instance, the salt-induced activation of MKK4 regulates the activity of MPK3, which increases the expression of stress-responsive genes such as NCED3 and RD29A [55][103]. The protein kinase SnRK2 family can be activated in both an ABA-dependent and -independent way. SnRK2.2, SnRK2.3, and SnRK2.6 are important for transducing the ABA signal, activating AREB/ABF TFs (ABA-responsive element binding factors), and regulating the downstream responsive gene [56][57][58][104,105,106].
