2. Definitions
The concomitant presence of typical angina and absence of significant stenosis on coronary angiography has been historically defined as “Cardiac Syndrome X” (CSX) due to uncertainty about its mechanism
[3]. The better understanding of this condition led to the replacement of the term “CSX” with microvascular angina (MVA)
[4], which encompasses patients with coronary vasomotor dysfunction consisting of microvascular spasm and/or impaired vasodilatation
[5][6][5,6]. While microvascular spasm can be inferred in patients with typical angina and ischemic electrocardiogram (ECG) changes during a spasm provocative test
[7], impaired vasodilator reserve due to coronary microvascular dysfunction (CMD) is characterized by a low coronary flow reserve (CFR)
[5]. Differently, a transient reduction in coronary blood flow (CBF) secondary to a spasm in the epicardial coronary arteries, previously referred to as “Prinzmetal” or “variant” angina, defines the condition of vasospastic angina (VSA)
[7,8,9,10]. The pathophysiological mechanisms of these conditions are addressed later in this [7][8][9][10]review.
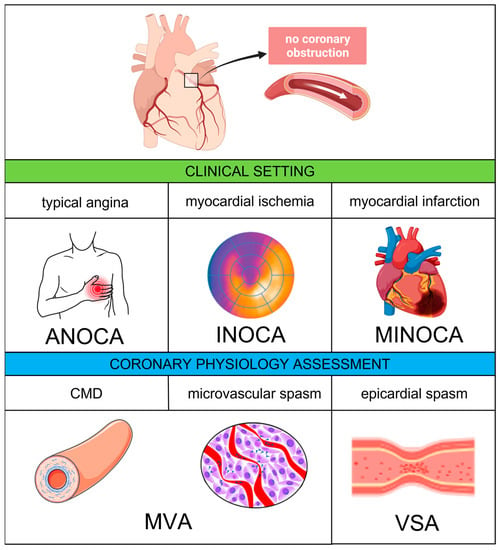
Recently, the term “ANOCA” has been coined to include all the clinical conditions determined by anginal symptoms without evidence of significant CAD on coronary angiography. When anginal symptoms are associated with documented myocardial ischemia, the term “INOCA” applies. Finally, in the presence of a universal criteria-defined myocardial infarction (MI) with no significant coronary stenosis and no other overt cause at presentation, the definition of “myocardial infarction with non-obstructive coronary artery” (MINOCA) can be used
[11]. A comprehensive diagram including the abovementioned definitions is provided by
Figure 1.
Figure 1. Coronary vascular dysfunction—clinical settings and pathophysiological mechanisms. Abbreviations: ANOCA, angina with non-obstructive coronary arteries; CMD, coronary microvascular dysfunction: INOCA, ischemia with non-obstructive coronary arteries; MINOCA, myocardial with non-obstructive coronary arteries; MVA, microvascular angina; VSA, vasospastic angina.
3. Epidemiology and Prognostic Implications
The absence of significant coronary obstruction is frequent in patients experiencing angina and myocardial ischemia. With regard to ANOCA, numerous data show that its prevalence ranges from 30% to 50% among patients undergoing coronary angiography
[1][12][13][14][1,12,13,14], with a female preponderance
[14][15][16][14,15,16]. Interestingly, these percentages seem to remain stable over time despite improvements in the definition and detection of this condition
[12]. Nevertheless, the correct characterization of symptoms has a central role, since the presence of typical angina is a predictor of significant CAD, while atypical symptoms are more often associated with the absence of coronary obstruction
[1][13][1,13]. When a non-invasive stress test confirms the presence of significant myocardial ischemia in patients with angina, the percentage of patients without significant CAD decreases
[1][17][1,17]. The prevalence of MINOCA has been reported to range between 2% and 10% of all Mis
[18][19][20][21][22][18,19,20,21,22], while coronary obstruction is found in more than 90%
[23]. Interestingly, women have a higher chance of a negative coronary angiography than men also in the setting of MI
[18].
Notably, none of these conditions is benign. In addition to impaired quality of life, several studies reported an increased risk of adverse events in patients with ANOCA and INOCA
[24][25][26][24,25,26]. The available data show a 1.3 higher risk of death and a 3–4-fold higher risk of hospitalization for cardiovascular events in ANOCA patients as compared with asymptomatic individuals
[14][25][14,25], while the Women’s Ischemia Syndrome Evaluation (WISE) study demonstrated a 10-year risk of all-cause death of 13% and 2.8% in patients with INOCA and in control healthy individuals, respectively
[27]. In MINOCA patients, the prognosis is influenced by the underlying mechanism and the extent of myocardial dysfunction. Overall, the risk of mortality in patients with MINOCA is estimated to be about 1%, 3% and 4% in hospital, at 1 year and at 3 years, respectively
[22][28][29][22,28,29]. Finally, the absence of significant coronary obstruction on coronary angiography may lead the treating physician to wrongly reassure patients or discontinue the medical therapy, with a potential negative impact on symptoms and quality of life.
4. Pathophysiology
Among the main pathophysiological mechanisms deemed responsible for ANOCA/INOCA and MINOCA, vascular spasm and CMD play an important role.
4.1. Epicardial and Microvascular Vasospasm
Epicardial vasospasm is a pathologic condition characterized by a transient reduction of CBF due to a spasm in the epicardial coronary arteries. This phenomenon is responsible for recurrent attacks of chest pain similar to those occurring in patients with obstructive CAD; however, these episodes often happen at rest rather than under exertion, and effort tolerance is generally preserved
[30]. While initially referred to as “Prinzmetal” or “variant” angina due to its differences from classical effort angina, the exact definition of the pathophysiology of this phenomenon led to the introduction of the term “VSA”.
The hyperreactivity of vascular smooth cells (VSCs) is thought to play a central role in the pathophysiology of VSA. VSCs’ contraction is mediated by the phosphorylation and dephosphorylation of myosin light chains (MLCs). MLC dephosphorylation, in particular, is inhibited by rho-kinase, which has been shown to be hyper-expressed in the spastic cells
[31][32][31,32]. This hypothesis is supported by preliminary studies suggesting that the use of rho-kinase inhibitors effectively prevents acetylcholine (Ach)-induced coronary spasms
[33]. The occurrence of anginal symptoms at night, when the vagal tone is higher, and the induction of spasm by Ach suggest that dysregulation in the autonomic nervous system may be involved as well in the pathophysiology of VSA
[8][34][8,34]. Other potential mechanisms include endothelial dysfunction with dysregulation of the nitric oxide synthases, magnesium deficiency, oxidative stress, inflammation and genetic polymorphisms
[8]. Coronary spasm often occurs at the level of an atherosclerotic plaque, probably because the endothelial dysfunction, leading to an imbalance between vasodilator and vasopressor stimuli
[35], can act as a trigger for local injury, ischemic damage and MI
[36]. However, even healthy vessels can be affected.
Several studies suggest that a spasm can also occur at the level of the microcirculation. Mohri et al. showed that lactate was produced during a spasm provocation test in patients with angina attack and ischemic ECG changes without significant epicardial coronary obstruction
[37]. Sun et al. confirmed these findings demonstrating the occurrence of myocardial ischemia (typical angina, ischemic ECG changes and lactate production) in patients undergoing the spasm provocative test without angiographic evidence of an epicardial spasm
[38]. These data support the hypothesis that, in the absence of an epicardial spasm, patients with chest pain and ECG changes during the spasm provocative test suffer from microvascular spasm. Of note, it should be considered that patients with non-diagnostic Ach-test results may be affected by CMD
[7]. Finally, one of the main characteristics of both epicardial and microvascular vasospasms is the resolution of symptoms due to vasodilation in response to nitrates.
4.2. Coronary Microvascular Dysfunction
CMD is a condition that is characterized by reduced CFR either due to increased minimal microvascular resistance (MVR) or high resting flow due to the altered non-endothelial-dependent vasomotion of the coronary microvasculature (<500 μm)
[39][40][41][42][39,40,41,42]. Based on the underlying mechanism, two clinical entities can be distinguished, structural and functional CMD. The main structural changes associated with CMD are the thickening of the arterioles’ wall with a reduction in the lumen area, the increase in perivascular fibrous tissue and the decrease in the vascular density (capillary rarefaction), leading to high MVR. Additionally, non-endothelial-dependent mechanisms such as the impaired relaxation of VSCs, higher susceptibility of VSCs to vasoconstrictor stimuli and abnormal autonomic activity may be involved
[43][44][43,44]. These abnormalities lead to an increase in the MVR and an insufficient increase in CBF under physiological stress, contributing to an imbalance between oxygen demand and supply, resulting in myocardial ischemia
[5][41][5,41].
The parameter that expresses the ability of the coronary microcirculation to increase CBF under stress conditions is the CFR, defined as the ratio of the CBF during maximal vasodilation to the corresponding value at rest. Unlike other parameters used to assess the hemodynamic significance of a coronary stenosis, such as fractional flow reserve (FFR) and instantaneous wave-free ratio (iFR), CFR provides complementary information about the overall functional status of the coronary circulation. There can be a correlation between FFR, iFR and CFR in certain cases; for example, if a coronary stenosis is severe enough to limit CBF, then FFR, iFR and CFR may all be found to be altered. By contrast, other conditions, such as the presence of collateral circulation or CMD, may alter this relationship. In the case of well-developed collaterals supplying adequate CBF to the affected territory, CFR may remain preserved even in the case of an FFR and/or iFR significant stenosis. Furthermore, an altered CFR may suggest CMD in symptomatic patients with non-hemodynamically significant coronary stenosis at FFR and/or iFR assessment
[45][46][45,46].
Typically, patients with CMD due to altered microvascular architecture present with low CFR and high MVR values. However, a proportion of patients with functional CMD may exhibit low CFR/low MVR and high resting CBF values. The mechanism responsible for this specific subset of CMD is still unclear, as this might be due to either a reduced myocardial efficiency or uncoupled CBF
[47]. Structural changes in the coronary microvascular have been shown to be more prevalent in patients with classical cardiovascular risk factors, including hypertension, hyperlipidemia, smoking and diabetes mellitus (DM). Moreover, they have been correlated with other conditions, including renal impairment, coronary atherosclerosis, ventricular hypertrophy, and other cardiomyopathies
[5][48][5,48].
While different findings on the coronary function tests (CFTs) may suggest that coronary microvascular spasm and CMD might represent two separated entities, an anomalous vasodilatory response has been proven in patients presenting the abovementioned risk factors and predisposition for structural abnormalities
[48], suggesting that both non-endothelial-dependent and endothelial-dependent alterations may coexist in patients with MVA. Importantly, the coexistence of CMD and VSA portends a worse prognosis.