Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Wendy Huang and Version 1 by Cody Criss.
Primary liver cancer is the third most common cause of cancer-related deaths worldwide. Risk factors for primary liver cancer include chronic viral hepatitis B and C infections, alcohol abuse, non-alcoholic fatty liver disease, and obesity. Surgical resection and/or transplantation is the mainstay treatment for candidates with primary liver tumors. However, minimally invasive, image-guided locoregional therapies have become an integral part of liver cancer treatment and management, depending on staging.
- primary liver cancer
- hepatocellular carcinoma
- intrahepatic cholangiocarcinoma
- incidence
- treatment
1. Introduction
Liver cancer constitutes one of the most common causes of malignancy worldwide, and rates for primary liver tumors are steadily rising in the United States [1,2][1][2]. The highest reported cases of liver cancer are in Eastern Asia and Middle Africa, and the incidence in men is roughly 2–4 times that of women [1]. Perhaps most alarmingly, liver cancer carries a high risk of mortality, with a 5-year survival rate of 6.5% [1]. Major risk factors have been identified for primary liver cancer, including chronic viral hepatitis B and C infections, alcohol abuse, non-alcoholic fatty liver disease, and obesity. There are two main types of primary liver cancers, including hepatocellular carcinoma and intrahepatic cholangiocarcinoma. In general, surgical resection and/or transplantation is the mainstay treatment for candidates with primary liver tumors. However, locoregional therapies, defined as minimally-invasive, image-guided procedures, have become an integral part of liver cancer treatment and management [3,4,5][3][4][5]. Depending on staging, image-guided locoregional therapies (iLRT), such as ablation (e.g., radiofrequency ablation, microwave ablation, cryoablation), transarterial embolization (TAE) or chemoembolization (TACE), or radioembolization (TARE) can be used as curative, neo-adjunctive, or palliative treatment regimens. The efficacy of these techniques is evaluated by follow-up imaging, via CT or MRI, and the gold standard tool for assessing treatment responses to these techniques is the Response Evaluation Criteria in Solid Tumors (RECIST) [6,7][6][7]. It is widely recognized that changes in tumor size, as measured by RECIST, can be used as surrogate endpoints for survival length, meaning that improvements in tumor size often correlate with longer survival times [7]. WithiIn the following manuscript, we provide is research, a brief overview of primary liver cancers and describe the treatment approaches of iLRT are provided, the rationales for treatment, and new emerging evidence for their use.
2. Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide, accounting for 80–90% of primary liver cancer cases [8,9][8][9]. The highest incidence rates are seen in sub-Saharan Africa and Southeast Asia, where viral risk factors, such as hepatitis B virus (HBV), are endemic [10]. In developed countries, HCC incidence is rising due to the increasing prevalence of non-alcoholic fatty liver disease, obesity, and diabetes [11]. In the United States, HCC incidence has tripled since the 1980s, accounting for up to 90% of primary liver cancers [12]. The epidemiology of HCC is complex and multifactorial, with risk factors including viral hepatitis, alcohol consumption, metabolic disorders, and exposure to hepatotoxic chemicals [9,10,13][9][10][13].
HCC is a heterogeneous disease with diverse histological subtypes and molecular characteristics. The most common histological subtype is well-differentiated HCC, which accounts for about 30% of cases. Poorly differentiated HCC, also known as hepatoblastoma-like HCC, is a rare, but aggressive, subtype that is associated with worse outcomes [14]. Other histological subtypes also exist, including fibrolamellar, scirrhous, and macrotrabecular-massive subtypes [14,15][14][15]. The tumor microenvironment plays a crucial role in HCC development and progression, with chronic inflammation, immune dysregulation, and fibrosis acting as key drivers [14]. Recent advances in molecular profiling technologies have identified new molecular subtypes of HCC, which may have clinical implications for patient stratification and treatment [16].
Prognosis of HCC varies depending on several factors, including tumor stage, liver function, and overall health status. HCC is a deadly disease, with a 5-year survival rate of less than 20% [17]. However, early detection and treatment can significantly improve outcomes. The Barcelona Clinic Liver Cancer (BCLC) staging system is widely used to classify HCC patients into different treatment categories (e.g., surgery, iLRT, and systemic treatment) based on tumor burden, liver function, and performance status (Figure 1) [18]. The classification system was recently updated in 2022, and it has been externally validated and endorsed by the Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) [18]. Patients with early-stage HCC (BCLC stage 0 or A) have better outcomes and are eligible for potentially curative treatments, such as surgical resection, liver transplantation, or ablation therapy. However, most patients present with advanced-stage disease (BCLC stage B or C), so they have limited treatment options. For patients with advanced-stage disease, systemic therapy is the standard of care, but the efficacy of these treatments is modest, and there is an urgent need for new therapeutic options. Patients with end-stage HCC (BCLC stage D) are typically managed with supportive care.
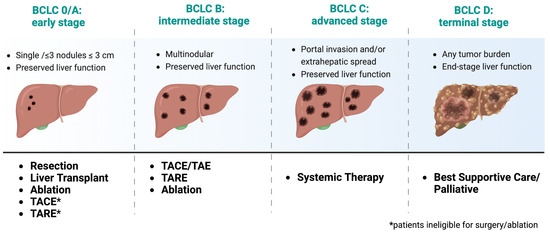
Figure 1. Treatment recommendations based on recent updates from the 2022 Barcelona Clinic Liver Cancer (BCLC) Guidelines [18]. Adapted from “Barcelona Clinic Liver Cancer (BCLC) Staging and Classification”, by BioRender.com (2023). https://app.biorender.com/biorender-templates, accessed on July 2023.
3. Intrahepatic Cholangiocarcinoma
Intrahepatic cholangiocarcinoma (ICCA) is a rare and aggressive cancer arising from the biliary tree within the hepatic parenchymal system [19]. ICCA exhibits traits of cholangiocyte differentiation, and it is likely to originate, mainly, from the epithelial cells that line the bile ducts, known as cholangiocytes [19,20][19][20]. Nevertheless, the tumors can also emerge from peribiliary glands and hepatocytes, depending on the location and underlying liver condition. It is the second most common type of primary liver cancer, after HCC carcinoma, accounting for roughly 10–15% of primary liver cancers [21,22][21][22]. Reports have also shown progressive increases in the incidence of ICCA worldwide [23,24,25][23][24][25]. However, the epidemiology of ICC remains complex and poorly understood due to its rarity and lack of population-based studies [25].
In the United States, the incidence of cholangiocarcinoma has almost tripled over the past three decades [26]. Similar to HCC, chronic liver disease, including cirrhosis and hepatitis B or C infection, is a significant risk factor for ICCA [19]. Other risk factors include exposure to certain chemicals, such as thorium dioxide and vinyl chloride, as well as inflammatory bowel disease [19,27,28][19][27][28]. There is also a strong association between cholangiocarcinoma and liver fluke parasitic infections within parts of Southeast Asia [19]. Prognosis of cholangiocarcinoma is poor, with a 5 year overall survival rate ranging from 25–31% and a recurrence rate ranging from 40–64% [29,30][29][30]. Even worse, ICCA is often beyond the limits of surgical therapy at the time of diagnosis, and the median survival time after treatment with chemoradiotherapy is only 10 months [31]. Surgical resection is the only potentially curative treatment for ICCA, but only a minority of patients are eligible for surgery due to the advanced stage of the disease at the time of diagnosis [32]. Therefore, for unresectable disease, candidates must rely on other non-surgical methods for disease management, such as iLRT or chemotherapy.
References
- Augustine, M.M.; Fong, Y. Epidemiology and Risk Factors of Biliary Tract and Primary Liver Tumors. Surg. Oncol. Clin. 2014, 23, 171–188.
- Makary, M.S.; Khandpur, U.; Cloyd, J.M.; Mumtaz, K.; Dowell, J.D. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers 2020, 12, 1914.
- Labib, P.L.; Davidson, B.R.; Sharma, R.A.; Pereira, S.P. Locoregional Therapies in Cholangiocarcinoma. Hepatic Oncol. 2017, 4, 99–109.
- Bosch, F.X.; Ribes, J.; Díaz, M.; Cléries, R. Primary Liver Cancer: Worldwide Incidence and Trends. Gastroenterology 2004, 127, S5–S16.
- Owen, M.; Makary, M.S.; Beal, E.W. Locoregional Therapy for Intrahepatic Cholangiocarcinoma. Cancers 2023, 15, 2384.
- Llovet, J.M.; Lencioni, R. MRECIST for HCC: Performance and Novel Refinements. J. Hepatol. 2020, 72, 288–306.
- Ko, C.-C.; Yeh, L.-R.; Kuo, Y.-T.; Chen, J.-H. Imaging Biomarkers for Evaluating Tumor Response: RECIST and Beyond. Biomark. Res. 2021, 9, 52.
- Center, M.M.; Jemal, A. International Trends in Liver Cancer Incidence Rates. Cancer Epidemiol Biomark. Prev 2011, 20, 2362–2368.
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13.
- Janevska, D.; Chaloska-Ivanova, V.; Janevski, V. Hepatocellular Carcinoma: Risk Factors, Diagnosis and Treatment. Open Access Maced. J. Med. Sci. 2015, 3, 732–736.
- Younossi, Z.M.; Henry, L. Epidemiology of Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma. JHEP Rep. 2021, 3, 100305.
- Flores, Y.N.; Datta, G.D.; Yang, L.; Corona, E.; Devineni, D.; Glenn, B.A.; Bastani, R.; May, F.P. Disparities in Hepatocellular Carcinoma Incidence, Stage, and Survival: A Large Population-Based Study. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1193–1199.
- Balogh, J.; Victor, D.; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P. Hepatocellular Carcinoma: A Review. J. Hepatocell. Carcinoma 2016, 3, 41–53.
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular Carcinoma (HCC): Epidemiology, Etiology and Molecular Classification. Adv. Cancer Res. 2021, 149, 1–61.
- Schlageter, M.; Terracciano, L.M.; D’Angelo, S.; Sorrentino, P. Histopathology of Hepatocellular Carcinoma. World J. Gastroenterol. 2014, 20, 15955–15964.
- Wu, Y.; Liu, Z.; Xu, X. Molecular Subtyping of Hepatocellular Carcinoma: A Step toward Precision Medicine. Cancer Commun. 2020, 40, 681–693.
- Brar, G.; Greten, T.F.; Graubard, B.I.; McNeel, T.S.; Petrick, J.L.; McGlynn, K.A.; Altekruse, S.F. Hepatocellular Carcinoma Survival by Etiology: A SEER-Medicare Database Analysis. Hepatol. Commun. 2020, 4, 1541–1551.
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693.
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65.
- Halder, R.; Amaraneni, A.; Shroff, R.T. Cholangiocarcinoma: A Review of the Literature and Future Directions in Therapy. Hepatobiliary Surg. Nutr. 2022, 11, 555–566.
- Ustundag, Y.; Bayraktar, Y. Cholangiocarcinoma: A Compact Review of the Literature. World J. Gastroenterol. 2008, 14, 6458–6466.
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 1073274817729245.
- Patel, T. Worldwide Trends in Mortality from Biliary Tract Malignancies. BMC Cancer 2002, 2, 10.
- Taylor-Robinson, S.; Toledano, M.; Arora, S.; Keegan, T.; Hargreaves, S.; Beck, A.; Khan, S.; Elliott, P.; Thomas, H. Increase in Mortality Rates from Intrahepatic Cholangiocarcinoma in England and Wales 1968–1998. Gut 2001, 48, 816–820.
- Van Dyke, A.L.; Shiels, M.S.; Jones, G.S.; Pfeiffer, R.M.; Petrick, J.L.; Beebe-Dimmer, J.L.; Koshiol, J. Biliary Tract Cancer Incidence and Trends in the United States by Demographic Group, 1999–2013. Cancer 2019, 125, 1489–1498.
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599.
- Tyson, G.L.; El-Serag, H.B. Risk Factors of Cholangiocarcinoma. Hepatology 2011, 54, 173–184.
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next Horizon in Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588.
- Shirono, T.; Niizeki, T.; Iwamoto, H.; Shimose, S.; Suzuki, H.; Kawaguchi, T.; Kamachi, N.; Noda, Y.; Okamura, S.; Nakano, M.; et al. Therapeutic Outcomes and Prognostic Factors of Unresectable Intrahepatic Cholangiocarcinoma: A Data Mining Analysis. J. Clin. Med. 2021, 10, 987.
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-Analysis. JAMA Surg. 2014, 149, 565–574.
- Sumiyoshi, T.; Shima, Y.; Okabayashi, T.; Negoro, Y.; Shimada, Y.; Iwata, J.; Matsumoto, M.; Hata, Y.; Noda, Y.; Sui, K.; et al. Chemoradiotherapy for Initially Unresectable Locally Advanced Cholangiocarcinoma. World J. Surg. 2018, 42, 2910–2918.
- Alvaro, D.; Gores, G.J.; Walicki, J.; Hassan, C.; Sapisochin, G.; Komuta, M.; Forner, A.; Valle, J.W.; Laghi, A.; Rizvi, S.I.; et al. EASL-ILCA Clinical Practice Guidelines on the Management of Intrahepatic Cholangiocarcinoma. J. Hepatol. 2023, 79, 181–208.
More
