Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Julien A. Delbrouck.
Steroidal (glycol)alkaloids S(G)As are secondary metabolites made of a nitrogen-containing steroidal skeleton linked to a (poly)saccharide, naturally occurring in the members of the Solanaceae and Liliaceae plant families. The genus Solanum is familiar to all of us as a food source (tomato, potato, eggplant), but a few populations have also made it part of their ethnobotany for their medicinal properties. The recent development of the isolation, purification and analysis techniques have shed light on the structural diversity among the SGAs family, thus attracting scientists to investigate their various pharmacological properties.
- Solanum
- steroid
- glycoalkaloid
1. Introduction
Nutrition is a vital act of communion with nature. Plants use the sun’s energy to transform minerals and gases into a cornucopia of organic molecules such as carbohydrates, lipids, terpenes, steroids, etc. Directly or indirectly, they supply all the energy and building blocks needed for the growth and maintenance of the human body. History shows that plants have been used for their medicinal properties in all civilizations and they continue today to be a source of bioactive molecules. The Solanum genus is comparatively a large one, encompassing over 1500 species and it is of particular importance in most of the world, as it includes popular food crops such as tomato (Solanum lycopersicum), potato (Solanum tuberosum) and eggplant (Solanum melongena). Two etymologies have been proposed: sol + anum (“of the Sun”) or solor + nus (“soothing”). Fittingly, it also contains various nightshade species used as folk medicine [1], such as the yellow-fruit nightshade (Solanum virginianum) used in Ayurveda medicine and the black nightshade (Solanum nigrum) used in both Western and Eastern traditional medicine. The genus Solanum is part of the Solanaceae family, which also includes the genera Capsicum (bell and chili peppers), Nicotiana (tobacco) as well as Datura, Mandragora and Atropa among others. It can thus be seen that many plants of this family are cultivated for their secondary metabolites like capsaicin and nicotine or are poisonous such as the deadly nightshade (Atropa belladonna).
At the interface between food, medicine, and poison, Steroidal Alkaloids (SAs) and their glycosidic versions are secondary metabolites that are especially prominent in the Solanum genus. Steroidal GlycoAlkaloids (SGAs) consist of a (poly)saccharide linked to a hydrophobic steroidal skeleton containing a nitrogen atom. They accumulate in different organs of the plants (leaves, roots, fruits, tubers, etc.) [2,3,4][2][3][4] and play a key role in the plant-environment interactions, in particular against bacterial and fungal attacks [5,6][5][6]. Their anti-nutrient properties and human toxicity are well-documented [3[3][7][8][9][10][11],7,8,9,10,11], while they more recently have attracted the attention of researchers for their wide range of pharmacological properties (anticancer, antibiotic, anti-inflammatory, etc.) [12,13,14,15][12][13][14][15]. For instance, Coramsine (SBP002) was an experimental chemotherapeutic drug composed of two steroidal glycoalkaloids (solamargine and solasonine) isolated from the species Solanum linneanum (devil’s apple) [16].
2. Steroidal (Glyco)Alkaloids: Classification
Steroidal alkaloids are nitrogenous derivatives of steroids and have been isolated and characterized from many organisms including animals (amphibians, sea sponges, etc.) and plants (Solanaceae, Liliaceae, etc.). They are important secondary metabolites with a wide range of potential therapeutic applications [145][17]. For instance, the steroidal alkaloid drug marketed Zytiga®, was approved in 2011 by the FDA for the treatment of metastatic castration-resistant prostate cancer [20][18]. The nitrogen atom is incorporated to the steroidal backbone either as heterocyclic ring, basic side chain or as a substituent at the C-3 position. Monomeric steroidal alkaloids are commonly classified into four groups based on their carbon heterocyclic skeleton: (1) Pregnane (C21); (2) Cyclopregnane (C24); (3) Cholestane (C27); (4) Others (Figure 21).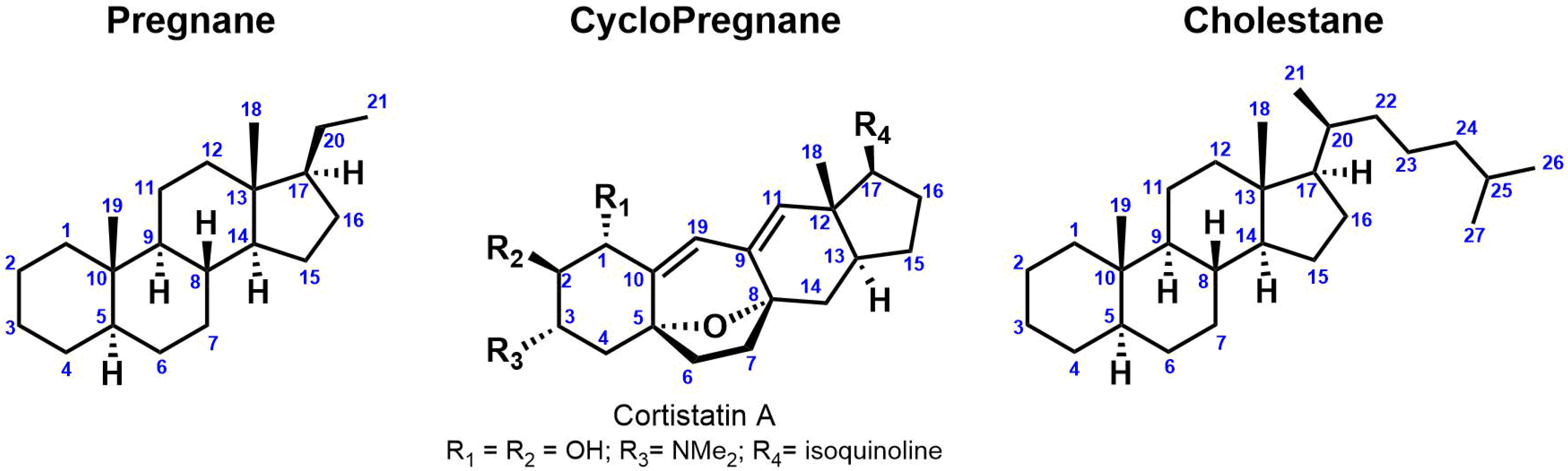
Figure 21.
Steroid categories according to their carbon framework.
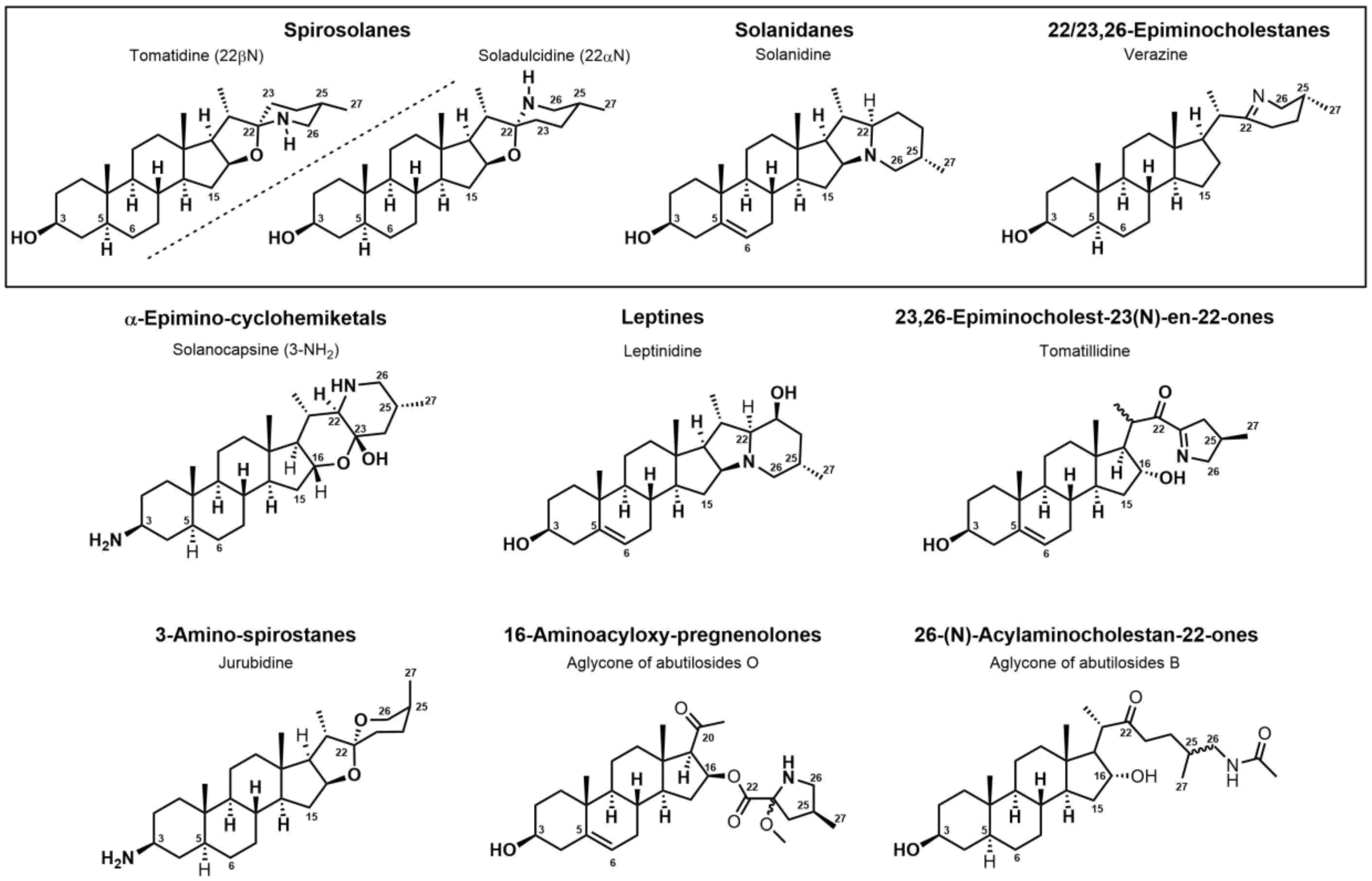
Figure 32.
Classification of steroidal alkaloids.
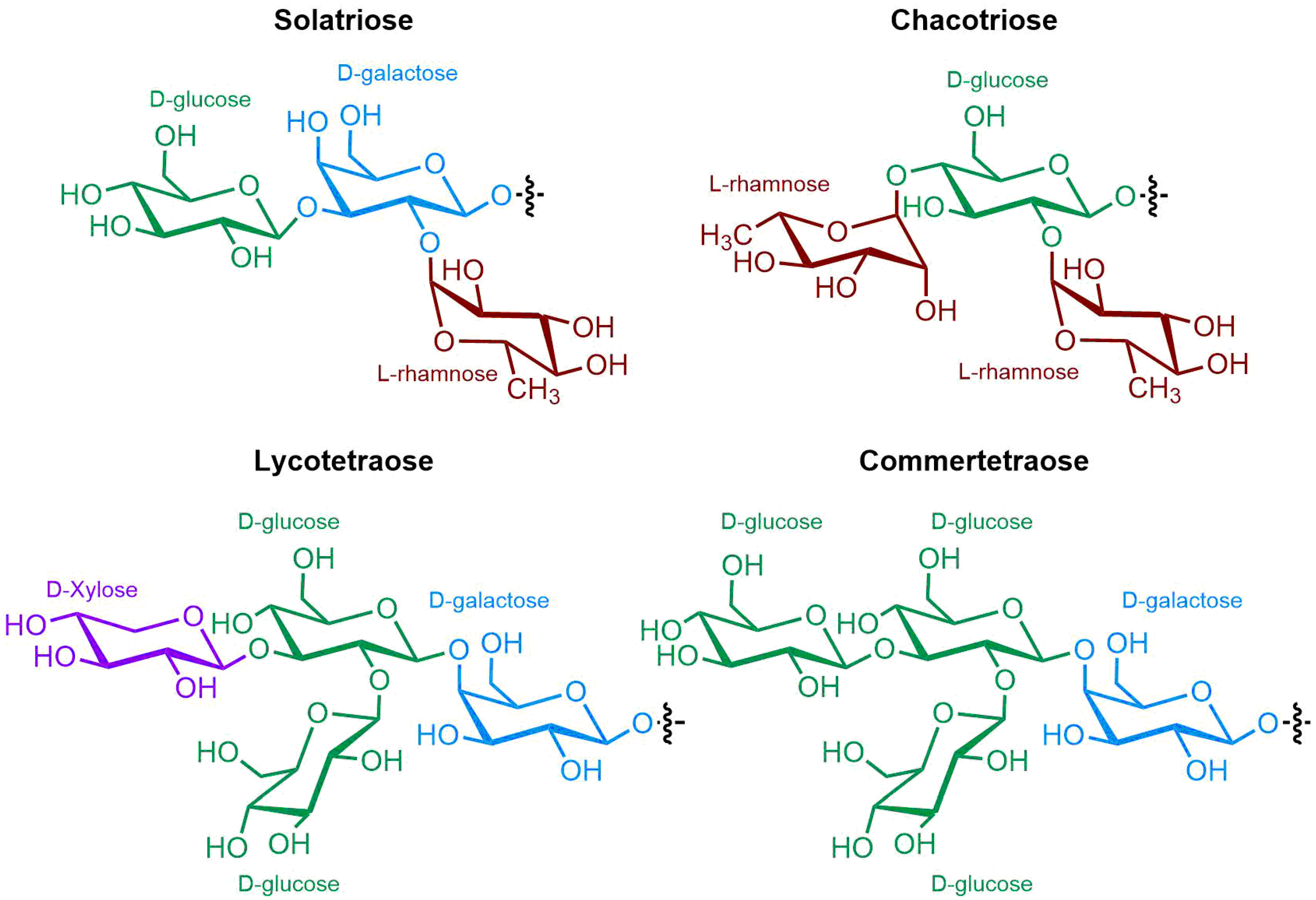
Figure 43.
Chemical structures of the main oligosaccharide units of Solanum SGAs.
References
- Jain, R.; Sharma, A.; Gupta, S.; Sarethy, I.P.; Gabrani, R. Solanum Nigrum: Current Perspectives on Therapeutic Properties. Altern. Med. Rev. 2011, 16, 78–85.
- Friedman, M.; Levin, C.E. Dehydrotomatine Content in Tomatoes. J. Agric. Food Chem. 1998, 46, 4571–4576.
- Friedman, M. Potato Glycoalkaloids and Metabolites: Roles in the Plant and in the Diet. J. Agric. Food Chem. 2006, 54, 8655–8681.
- Bednarz, H.; Roloff, N.; Niehaus, K. Mass Spectrometry Imaging of the Spatial and Temporal Localization of Alkaloids in Nightshades. J. Agric. Food Chem. 2019, 67, 13470–13477.
- Tingey, W.M. Glycoalkaloids as Pest Resistance Factors. Am. Potato J. 1984, 61, 157–167.
- Dahlin, P.; Muller, M.C.; Ekengren, S.; McKee, L.S.; Bulone, V. The Impact of Steroidal Glycoalkaloids on the Physiology of Phytophthora Infestans, the Causative Agent of Potato Late Blight. Mol. Plant Microbe Interact. 2017, 30, 531–542.
- Friedman, M. Tomato Glycoalkaloids: Role in the Plant and in the Diet. J. Agric. Food Chem. 2002, 50, 5751–5780.
- Fogelman, E.; Oren-Shamir, M.; Hirschberg, J.; Mandolino, G.; Parisi, B.; Ovadia, R.; Tanami, Z.; Faigenboim, A.; Ginzberg, I. Nutritional Value of Potato (Solanum tuberosum) in Hot Climates: Anthocyanins, Carotenoids, and Steroidal Glycoalkaloids. Planta 2019, 249, 1143–1155.
- Chowanski, S.; Winkiel, M.; Szymczak-Cendlak, M.; Marciniak, P.; Manczak, D.; Walkowiak-Nowicka, K.; Spochacz, M.; Bufo, S.A.; Scrano, L.; Adamski, Z. Solanaceae Glycoalkaloids: Alpha-Solanine and Alpha-Chaconine Modify the Cardioinhibitory Activity of Verapamil. Pharm. Biol. 2022, 60, 1317–1330.
- EFSA Panelon Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; et al. Risk Assessment of Glycoalkaloids in Feed and Food, in Particular in Potatoes and Potato-derived Products. EFS2 2020, 18, e06222.
- Smith, D. Potato Glycoalkaloids: Some Unanswered Questions. Trends Food Sci. Technol. 1996, 7, 126–131.
- Friedman, M.; Lee, K.-R.; Kim, H.-J.; Lee, I.-S.; Kozukue, N. Anticarcinogenic Effects of Glycoalkaloids from Potatoes against Human Cervical, Liver, Lymphoma, and Stomach Cancer Cells. J. Agric. Food Chem. 2005, 53, 6162–6169.
- Al Sinani, S.S.S.; Eltayeb, E.A. The Steroidal Glycoalkaloids Solamargine and Solasonine in Solanum Plants. S. Afr. J. Bot. 2017, 112, 253–269.
- Sbhatu, D.B.; Abraha, H.B. Preliminary Antimicrobial Profile of Solanum incanum L.: A Common Medicinal Plant. Evid. Based Complement. Alternat. Med. 2020, 2020, 3647065.
- Kenny, O.M.; McCarthy, C.M.; Brunton, N.P.; Hossain, M.B.; Rai, D.K.; Collins, S.G.; Jones, P.W.; Maguire, A.R.; O’Brien, N.M. Anti-Inflammatory Properties of Potato Glycoalkaloids in Stimulated Jurkat and Raw 264.7 Mouse Macrophages. Life Sci. 2013, 92, 775–782.
- Van der Most, R.G.; Himbeck, R.; Aarons, S.; Carter, S.J.; Larma, I.; Robinson, C.; Currie, A.; Lake, R.A. Antitumor Efficacy of the Novel Chemotherapeutic Agent Coramsine Is Potentiated by Cotreatment With CpG-Containing Oligodeoxynucleotides. J. Immunother. 2006, 29, 134–142.
- Dey, P.; Kundu, A.; Chakraborty, H.J.; Kar, B.; Choi, W.S.; Lee, B.M.; Bhakta, T.; Atanasov, A.G.; Kim, H.S. Therapeutic Value of Steroidal Alkaloids in Cancer: Current Trends and Future Perspectives. Int. J. Cancer 2019, 145, 1731–1744.
- Xiang, M.-L.; Hu, B.-Y.; Qi, Z.-H.; Wang, X.-N.; Xie, T.-Z.; Wang, Z.-J.; Ma, D.-Y.; Zeng, Q.; Luo, X.-D. Chemistry and Bioactivities of Natural Steroidal Alkaloids. Nat. Prod. Bioprospect. 2022, 12, 23.
- Eich, E. Solanaceae and Convolvulaceae: Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2008.
- Ono, M.; Takara, Y.; Egami, M.; Uranaka, K.; Yoshimitsu, H.; Matsushita, S.; Fujiwara, Y.; Ikeda, T.; Nohara, T. Steroidal Alkaloid Glycosides, Esculeosides C and D, from the Ripe Fruit of Cherry Tomato. Chem. Pharm. Bull. 2006, 54, 237–239.
- Yoshimitsu, H.; Nishida, M.; Nohara, T. Steroidal Glycosides from the Fruits of Solanum abutiloides. Phytochemistry 2003, 64, 1361–1366.
- Heftmann, E. Biogenesis of Steroids in Solanaceae. Phytochemistry 1983, 22, 1843–1860.
- Akiyama, R.; Watanabe, B.; Nakayasu, M.; Lee, H.J.; Kato, J.; Umemoto, N.; Muranaka, T.; Saito, K.; Sugimoto, Y.; Mizutani, M. The Biosynthetic Pathway of Potato Solanidanes Diverged from That of Spirosolanes Due to Evolution of a Dioxygenase. Nat. Commun. 2021, 12, 1300.
- Sonawane, P.D.; Jozwiak, A.; Panda, S.; Aharoni, A. ‘Hijacking’ Core Metabolism: A New Panache for the Evolution of Steroidal Glycoalkaloids Structural Diversity. Curr. Opin. Plant Biol. 2020, 55, 118–128.
- Cardenas, P.D.; Sonawane, P.D.; Pollier, J.; Vanden Bossche, R.; Dewangan, V.; Weithorn, E.; Tal, L.; Meir, S.; Rogachev, I.; Malitsky, S.; et al. GAME9 Regulates the Biosynthesis of Steroidal Alkaloids and Upstream Isoprenoids in the Plant Mevalonate Pathway. Nat. Commun. 2016, 7, 10654.
- Nohara, T.; Ono, M.; Ikeda, T.; Fujiwara, Y.; El-Aasr, M. The Tomato Saponin, Esculeoside A. J. Nat. Prod. 2010, 73, 1734–1741.
More
