You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Christos Mikropoulos and Version 2 by Conner Chen.
Endometrial cancer (EC) and cervical cancer (CC) are common malignancies in women in clinical practice. More uncommon non-ovarian malignancies, such as vulval cancer (VC), are also becoming more prevalent in women of all ages. According to The Cancer Genome Atlas (TCGA) Research Network, ECs can be classified into four groups according to their genetic and molecular information.
- endometrial cancer
- cervical cancer
- vulval cancer
1. Endometrial Cancer
According to The Cancer Genome Atlas (TCGA [1][61]) Research Network, ECs can be classified into four groups according to their genetic and molecular information (Figure 1):
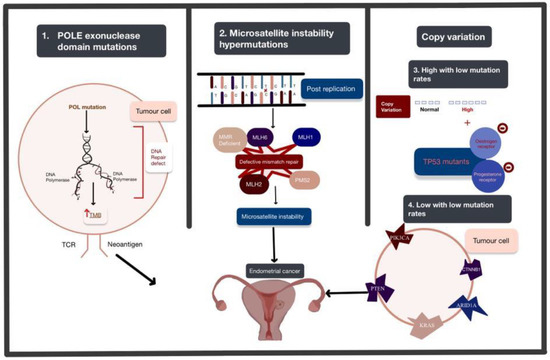
Figure 1. The four genomic alterations expressed in endometrial cancer (EC): POLE exonuclease domain mutations, microsatellite instability hypermutated, copy-number high with low mutation rate, and copy-number low with low mutation rate. The arrows used in this figure depict the order of the progression of these mutations from genetic alteration to the development of EC. Abbreviations: POLE: polymerase Epsilon; POL: polymerase; TMB: tumour mutational burden; TCR: T cell receptor.
Polymerase Epsilon (POLE) ultra-mutated: POLE encodes central catalytic and proofreading subunits of DNA polymerase epsilon involved in leading-strand DNA replication. This subtype is characterised by POLE exonuclease domain mutations (EDMs) mostly in hotspot regions, more than 20% C > A transversions and unusually high mutation rates (232 × 10−6 mutations per Mb). The exonuclease proofreading function locates and replaces erroneous bases in the daughter strand through complementary pairing and ensures a low mutation rate. In endometrial carcinomas, mutations in DNA polymerases inactivate or suppress the proofreading abilities and increase replicative error rates [2][62]. It is identified in less than 10% [3][63] of ECs and is associated with aggressive histopathological features but high 5-year recurrence-free survival of around 90% [4][64].
Microsatellite instability hypermutated (MSI-H): MSI arises from alteration of the MLH1, MSH2, MSH6 and PMS2 genes involved in the post-replicative DNA mismatch repair system. MMR corrects DNA mismatches generated during replication, thereby preventing mutations from becoming permanent [5][65] in dividing cells, and its defects increase the spontaneous mutation rate [6][66]. MMR deficiencies can result from inheritance (as in Lynch syndrome), somatic mutations or epigenetic alteration.
The copy-number high with low mutation rate subgroup is characterised by significantly reoccurring amplifications or deletion regions, frequent mutation of TP53, and low oestrogen and progesterone receptor expression. This subgroup shares the same features as basal-like breast cancer and serous ovarian carcinoma.
The copy-number low with low mutation rate subgroup includes the rest of the tumours that do not belong to other groups and are frequently associated with mutations in PTEN, CTNNB1, PIK3CA, ARID1A and KRAS [7][67]. This subtype appears to share a pathogenesis with colorectal tumours [8][68].
2. Cervical Cancer
The Cancer Genome Atlas (TCGA) Research Network discovered novel amplifications [9][69] in immune targets CD274/PD-L1 and PDCD1LG2/PD-L2 and the BCAR4 lncRNA in CCs (Figure 2).
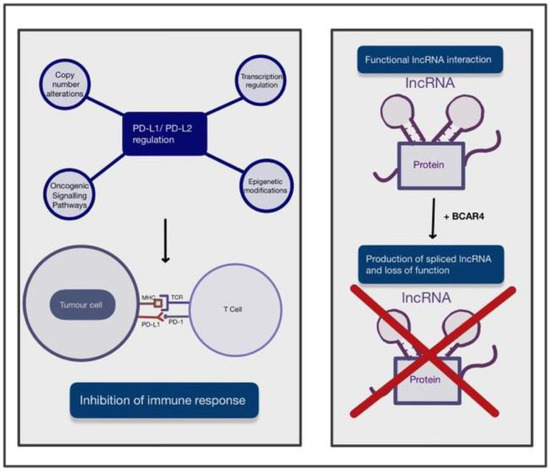
Figure 2. A figure depicting the two main novel amplications in the development of cervical cancer (CC). These include the upregulation of PD-L1/PD-L2 and BCAR4 expression and its role in disrupting the interaction of IncRNA with DNA, RNA and protein complexes. The arrows used in this figure depict the process of these novel amplifications and their effect on immune response or function. Abbreviations: PD-L1: programmed death ligand 1; PD-L2: programmed death ligand 2; MHC: major histocompatibility complex; TCR: tumour cell receptor; BRCA4: breast cancer anti-oestrogen resistance 4.
Programmed cell death ligand 1 (PD-L1) is a receptor mainly expressed on tumour cells and myeloid cells [10][70]. PD-L1 binds to receptor programmed cell death protein 1 (PD-1) on activated T cells and inhibits the immune response [11][71] towards tumour cells. PD-L2 is also a ligand of PD-1 and mainly present on antigen-presenting cells [12][72] and has an overlapping role with PD-L1. The regulation mechanisms of PD-L1/PD-L2 expression are complex and not yet fully understood. PD-L1 expression in tumour cells is regulated by various oncogenic signalling pathways and transcriptional and post-transcriptional factors [13][73]. Oncogenic factors that induce PD-L1 expression include the accumulation of oncogenic PIK3CA mutations and loss-of-function mutations of the negative regulator in the PI3K/Akt/mTOR pathway. In addition to these, there are also activation of the JAK/STAT pathway and the mitogen-activated protein kinase (MAPK) pathway by genetic mutations and growth factors [13][73]. Transcription factors, such as HIF-1, STAT3 and NF-κB, can bind to PD-L1 gene promoters, resulting in upregulation of its expression [13][73]. Finally, post-transcriptional regulation can be disrupted when microRNAs bind to mRNA, resulting in translational repression or alteration [13][73]. PD-L1 could play a significant role in the future management of CC, as HPV-positivity has been strongly correlated with an increase in PD-1 expression. This has been associated with an improved prognosis and a reduced chance of locoregional recurrence in HPV-positive cancers [14][74]. Additionally, a correlation has been found between high expression of PD-L1 and increased radiosensitivity, which may contribute to the improved prognostic rates seen in patients with high PD-L1 expression compared to patients with low PD-L1 expression [15][75]. Copy-number alterations at the 9p24.1 chromosomal region, transcriptional regulation by interferon factors, oncogenic signalling pathways, and epigenetic modifications by enzymes such as histone deacetylases may all contribute to regulation of PD-L1/PD-L2 [13][73]. In the case of CC, it has been shown that PD-L1 is significantly upregulated in productive HPV infection of the cervix.
Non-coding RNAs (ncRNAs) are RNAs that do not translate into a protein but are responsible for epigenetic regulation [16][76] at transcriptional and post-transcriptional levels, such as histone modification, DNA methylation and transcriptional silencing. NcRNAs can be categorised by size, and those longer than 200 nucleotides [17][77] are long ncRNAs (lncRNAs). LncRNAs could drive many important pathological cancer phenotypes [18][78] through their interactions with other molecules, such as DNA, protein and RNA. For instance, chromatin-bound lncRNAs can regulate gene expression; lncRNA interactions with multiple proteins can promote or impair the assembly of protein complexes; and lncRNAs can recruit proteins involved in mRNA metabolism. The breast cancer anti-oestrogen resistance 4 (BCAR4) gene was previously only studied in the context of breast cancer. BCAR4 produces a spliced lncRNA that is inversely associated with the development of resistance to anti-oestrogens in breast cancer cells and poor disease-free survival for recurrent breast cancer. Therefore, lncRNA BCAR4 was considered to play an important role in the metastasis and tamoxifen-resistance of breast cancer [19][79]. Over the years, more published evidence has suggested that the prognostic significance of BCAR4 also applies to gastrointestinal malignancy, breast cancer, osteosarcoma and various other cancer types [20][80]. It has been reported that BCAR4 expression was significantly upregulated in CC tissue and that patients with high BCAR4 expression showed worse survival outcomes. Moreover, overexpression of BCAR4 remarkably promoted the proliferation and motility of CC cells and the epithelial-mesenchymal transition process, while silencing BCAR4 had the reverse effect [21][81].
3. Vulval Cancer
VCs can develop via two major pathogenetic pathways [22][23][82,83], with 30–60% of vulvar tumours being related to HPV infection and the rest having no association with HPV. Both genetic mutations, such as TP53 mutation, and HPV infection can result in similar molecular effects [24][84], namely, inactivation of p53 and escape from apoptosis. Disruption of the TP53 tumour suppressor gene by point mutations plays a crucial role in VC, with the majority of mutations being single-point mutations in the TP53 core DNA-binding domain [25][26][85,86]. The p53 protein is essential for cellular responses under stress, such as DNA damage, hypoxia and oncogene activation. During cellular stresses, p53 binds to DNA as a tetramer by sequence matching and results in gene transcriptional regulation. This process leads to key cellular processes, such as DNA repair, cell-cycle arrest, senescence and apoptosis [27][87]. Thus, p53 is essential for tumour suppression and can be a potential target for cancer therapy [28][88].
Other than TP53 mutation as described in Figure 3, it has been reported that PD-L1 expression in squamous cell carcinoma of the vulva could be observed in 32.9% of patients, and its expression in peritumoural immune cells was confirmed in 91.4% of patients [29][89]. PD-L1 expression upregulation as described in Figure 3, has been suggested to be important for other genomic alterations or modifications described in vulval cancer.
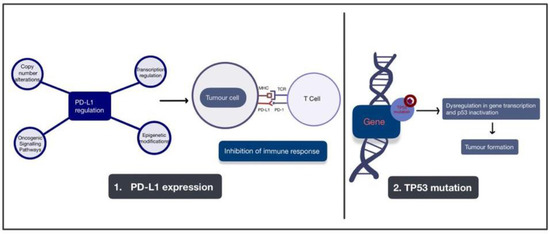
Figure 3. A figure depicting the two major pathogenic pathways involved with the development of vulval cancer. These pathways are the upregulation of PD-L1 expression and mutation of the TP53 gene. Arrows are used to depict the development of tumour formation as a result of these alterations. Abbreviations: PD-L1: programmed death ligand 1; PD-L2: programmed death ligand 2; MHC: major histocompatibility complex; TCR: tumour cell receptor; TP53: tumour protein 53.
Given the extensive knowledge of genomic alterations in EC, CC and VC, it is important that clinicians understand the molecular mechanisms underlying these cancers and use this knowledge to guide approaches to treatment whilst considering approaches to treatment, as well as how these alterations can affect disease progression and treatment efficacy.
