- antiplatelet therapy
- de-escalation
- acute coronary syndrome
- platelet function testing
- genetic testing
- P2Y12
1. Role of Platelet Function Testing in Assessing P2Y12 Inhibitor Therapy
2. The Use of P2Y12 Inhibitors in Acute Coronary Syndrome
3. Genetic Testing-Based P2Y12 De-Escalation Strategy
| Study | TALOS-AMI Trial | HOST-REDUCE-POLYTECH-ACS | TAILOR-PCI | TOPIC | TROPICAL-ACS | - |
|---|---|---|---|---|---|---|
| First author | Park | Kim | Pereira | Cuisset | Sibbing | Ueno |
| Publication year | 2021 | 2020 | 2020 | 2017 | 2017 | 2016 |
| Number of patients | 2697 | 2338 | 5302 | 646 | 2610 | 136 |
| De-escalation strategy | Uniform unguided de-escalation | Uniform unguided de-escalation | Genotype-guided therapy | Uniform unguided de-escalation | Guided by platelet function testing | Uniform unguided de-escalation |
| Primary outcome | NACE (CVD + MI + Stroke + Bleeding) | NACE (Death + MI + ST + SRI + Bleeding) | CVD + MI + ST + RR + Stroke | CVD + UR + Stroke + Bleeding | CVD + MI + Stroke + Bleeding | PRU |
| Definition of bleeding (Primary/Secondary) | BARC | BARC | BARC/TIMI | TIMI/BARC | BARC | BARC/TIMI |
| Treatment used before de-escalation | Ticagrelor + Aspirin | Prasugrel + Aspirin | Ticagrelor + Aspirin | Ticagrelor or Prasugrel + Aspirin | Prasugrel + Aspirin | Prasugrel + Aspirin |
| Treatment used after de-escalation | Clopidogrel + Aspirin | Prasugrel + Aspirin | Clopidogrel + Aspirin | Clopidogrel + Aspirin | Clopidogrel + Aspirin | Clopidogrel + Aspirin |
| Clopidogrel (Experimental/Control) (%) | 100/0 | - | 15/99 | 100/0 | 100/0 | 100/0 |
| Prasugrel(Experimental/Control) (%) | 0/100 | 100/100 | - | 56/59 | 0/100 | 0/100 |
| Ticagrelor (Experimental/Control) (%) | 0/100 | - | 85/1 | 44/42 | - | - |
| Result | Significant decrease in bleeding risk | Reduced risk of NACE | No significant results | Reduced risk of bleeding | No significant results | Increase in PRU |
4. Platelet Function Testing-Based P2Y12 De-Escalation Strategy
5. Trials with uniform P2Y12 De-Escalation Strategy
Several trials have investigated de-escalation protocols for P2Y12 treatment without considering patient-specific genetic or platelet function data. These trials compared long-term, potent DAPT to protocols that switched patients from potent inhibitors to clopidogrel after a predetermined period.
The TOPIC trial (Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment for Acute Coronary Syndromes) randomized 646 ACS patients on DAPT to either switch to clopidogrel or continue the newer P2Y12 inhibitor one month after PCI. The primary endpoint of cardiovascular death, myocardial infarction, stroke, or stent thrombosis occurred in 26.3% of patients in the unswitched group and 13.4% of the switched group (HR: 0.48, [95% CI: 0.34 - 0.68], P<0.01). No significant difference in ischemic endpoints was reported between the two groups, while bleeding occurred in 4.0% of patients in the switched DAPT and 14.9% in the unswitched DAPT group (HR: 0.30, [95% CI: 0.18 - 0.50], P<0.01) [14].
The HOST-REDUCE-POLYTECH-ACS trial randomized 2,338 ACS patients on DAPT to either continue their current P2Y12 inhibitor dose of prasugrel (10 mg) or receive a lower dose of prasugrel (5 mg). The primary endpoint of a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, or ischemic stroke occurred in 7.2% of patients in the de-escalation group and 10.1% of patients in the standard care group (P-non-inferiority<0.0001, HR: 0.70, [95% CI: 0.52 - 0.92], P-equivalence=0.012). There was no increase in ischemic risk in the de-escalation group compared with the conventional group (HR: 0.76, [95% CI: 0.40 - 1.45], P=0.40), and the risk of bleeding events was significantly decreased (HR: 0.48, [95% CI: 0.32 - 0.73], P=0.0007) [15].
The TALOS-AMI trial randomized 2,697 patients on DAPT to either undergo de-escalation to clopidogrel with aspirin or continue DAPT with ticagrelor. The primary endpoint of net adverse clinical events (NACE), including cardiovascular death, myocardial infarction, stroke, and BARC 3 or 5 bleeding, occurred in 4.7% of patients in the de-escalation group and 8.3% of patients in the control group (HR: 0.58, [95% CI: 0.38 - 0.87], P=0.009), with a significant decrease in bleeding (HR: 0.52, [95% CI: 0.35 - 0.77], P=0.001) and no increase in ischemic events [16].
Ueno et al. randomized 136 ACS patients on DAPT to either undergo de-escalation to clopidogrel with aspirin or continue DAPT with prasugrel. The primary endpoint was the mean P2Y12 reaction unit (PRU) at week 6, which was significantly lower in the continued group relative to the switched group (140.7 and 183.0, respectively; P=0.001) [17].6. Comparison of Approaches
Comparing the effectiveness of the three de-escalation approaches to P2Y12 de-escalation, including PFT guidance, genetic testing guidance, and uniform de-escalation without laboratory guidance, is challenging due to variations in patient populations, follow-up durations, and endpoints among the trials. Notably, none of these individual trials found a significant reduction in major bleeding, MACE, or net clinical benefit. However, their results supported that protection against ischemic events is not compromised with de-escalation compared to long-term potent P2Y12 treatment.
Risk and benefit of de-escalation related to other strategies antiplatelet strategies were assessed in multiple recent analyses. A recent network meta-analysis aimed to compare the efficacy and safety of different approaches linking standard long-term DAPT with potent P2Y12 antagonists to strategies based on earlier aspirin cessation and potent P2Y12 inhibitor monotherapy after coronary intervention [18]. Ten randomized controlled trials with a total of 42,511 participants were included. They compared four different strategies for abating DAPT: PFT-based P2Y12 de-escalation, genetic testing-based P2Y12 de-escalation, uniform unguided P2Y12 de-escalation, and P2Y12 monotherapy, including ticagrelor monotherapy and clopidogrel arms, which allowed a broader context to relate the efficacy and safety of abatement strategies.
The authors found that both P2Y12 inhibitor de-escalation and P2Y12 inhibitor monotherapy reduce ischemic events and all bleeding (including major and minor events) among PCI-treated ACS patients. However, the different severity of bleeding was differently affected by the abatement strategies. With ticagrelor monotherapy, both major and minor bleeding event risk was significantly reduced, while with de-escalation, only the risk of minor bleeding was significantly reduced.
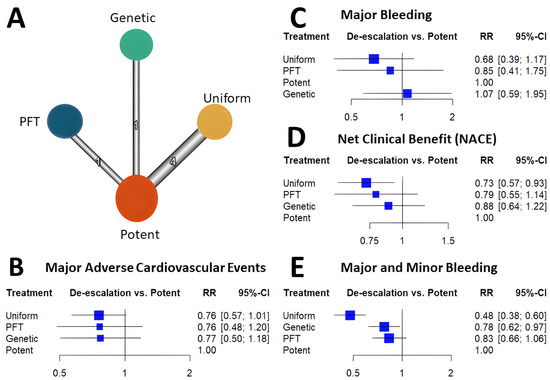
Among the de-escalation strategies, uniform de-escalation exhibited the highest reduction in bleeding, followed by genetic testing-guided de-escalation, while PFT-guided de-escalation did not show any significant reduction in bleeding (Figure 1). These trends reached significant levels for all bleeding and minor bleeding, but regarding major bleeding, none of the individual de-escalation strategies nor the cumulative estimate of the de-escalation trials reflected a significant reduction. While results of the bleeding risk reduction remained behind expectations for de-escalation strategies, an unexpected benefit was unveiled. Contrary to the anticipated trade-off of accepting a certain increase in ischemic risk, all three P2Y12 inhibitor de-escalation strategies resulted in a similarly lower rate of ischemic events (Figure 2). As these trials were not powered to assess individual endpoints, the cumulative analysis of more than 10,000 randomized patients reflected a highly significant 24% reduction of MACE without signs of major heterogeneity among the trials. Similarly, in net clinical benefit analyses, a significant 22% reduction of adverse event risk was found.
In an extensive network meta-analysis conducted by Kuno et al., aimed to assess the efficacy and safety of various dual antiplatelet therapy (DAPT) approaches. Employment of broader inclusion criteria, permitted a higher number of trials with less stringent requirements regarding de-escalation. The analysis incorporated 19 randomized controlled trials, totaling 69,746 patients, and evaluated six distinct DAPT strategies, including aspirin and clopidogrel, aspirin and low-dose prasugrel, aspirin and standard-dose prasugrel, aspirin and ticagrelor,as well as unguided de-escalation strategy, and guided selection strategy. Although this approach may facilitate a better understanding of de-escalation within a broader range of therapeutic options, it also carries the risk of network results being influenced or dominated by indirect comparisons. Results of Kuno et al.'s findings, were in agreement, indicating that unguided de-escalation was associated with a reduced risk of major adverse cardiovascular events (MACE) when compared to DAPT regimens [19]. Researcher's further analyses revealed no significant difference in MACE risk between guided and unguided strategies, but all studies demonstrated similar reductions that reached statistical significance due to the larger cumulative number of patients included in unguided de-escalation trials.
While ischemic event outcomes suggested a similar benefit for de-escalation with or without laboratory guidance, bleeding rates presented a more heterogeneous picture. A key distinction between researchers's analysis and that of Kuno et al. is that the latter grouped the TROPICAL-ACS and POPULAR-GENETIC trials in the same category. The notable increase in major bleeding in the latter trial, despite significant reductions in major and minor bleeding with genetictesting-based de-escalation, remains unexplained. Researchers believe this discrepancy justifies not grouping these two trials together.
It is essential to note that none of the trials were designed to demonstrate differences in MACE or major bleeding, but rather to establish non-inferiority based on composite endpoints. Complementing Kuno et al.'s analysis, researchers demonstrated a significant improvement in net clinical benefit with de-escalation strategies. Researchers concur that both guided de-escalation approaches resulted in a higher number of prasugrel treatments in the de-escalation arm, which may explain the less pronounced reduction in major and minor bleeding rates. This observation, combined with cost and logistical concerns, renders unguided de-escalation a more attractive option [19].
Researchers lack a clear mechanistic explanation for the risk reduction, but together with the findings of minor bleeding rate, it has been hypothesized that reduction of these nuisance events may have permitted a more tolerable treatment with higher compliance. If this hypothetical higher adherence translated to the observed clinical benefit, however, researchers lack conclusive data [18]. The rate of bleeding events may also be influenced by additional factors. Both genetic testing and PFT were incorporated into the de-escalation strategies to implement pharmacokinetic-based risk stratification for identifying patients at the highest risk. However, the practical application of this strategy led to approximately 40% of patients in the individualized treatment arm receiving clopidogrel. A selection strategy resulting in a higher rate of potent treatment might be the reason why these trials' observed bleeding risk reduction fell short of expectations. It has been suggested that platelet function measurements' negative predictive values are excellent, potentially providing a valuable tool for identifying patients who can safely remain on clopidogrel therapy. For instance, in a group of ACS patients with access to more potent antiplatelet drugs, continuing clopidogrel therapy may be non-inferior to switching to prasugrel or ticagrelor. However, the positive predictive values of platelet function measurements are mostly fair or poor. While platelet function tests assess residual platelet reactivity, the connection between ischemic risk and genetic predisposition may be even weaker, which could explain the discrepancies in these trials.
Cumulative analyses of P2Y12 inhibitor de-escalation studies demonstrated significant benefits in MACE, NACE, and major + minor bleeding, with slightly greater benefits observed in the uniform studies. However, major bleeding did not show a significant reduction; it was more prevalent in uniform studies, followed by PFT de-escalation strategies, and lastly, genetic testing de-escalation, which exhibited a lesser trend of major bleeding reduction. These results might be attributed to the long-term prasugrel treatment in the de-escalation arms (40%) of both PFT and genetic testing de-escalation, which can impact clinical outcomes, particularly bleeding events. Additionally, these results suggest that risk assessment with PFT may be more precise compared to metabolizer status. Nonetheless, further studies will be necessary to support these assumptions.
Most trials demonstrated trends for improvement concerning these endpoints. A cumulative analysis resulted in a significant reduction in all three endpoints (Figure 1).
In summary, network analyses suggest that uniform unguided de-escalation may be an effective strategy for reducing potent P2Y12 antagonist-based DAPT after coronary intervention (Figure 2). However, this approach might be associated with an increased risk of ischemic events, as it does not consider each patient's individual bleeding and ischemic risk to select the optimal approach for DAPT abatement.
Overall, these network analyses suggest that uniform unguided de-escalation may be an effective strategy for abating potent P2Y12 antagonist-based DAPT after coronary intervention (Figure 2). However, this approach may be associated with an increased risk of ischemic events since it does not take into consideration the individual patient's bleeding and ischemic risk in order to select the optimal approach for DAPT abatement.
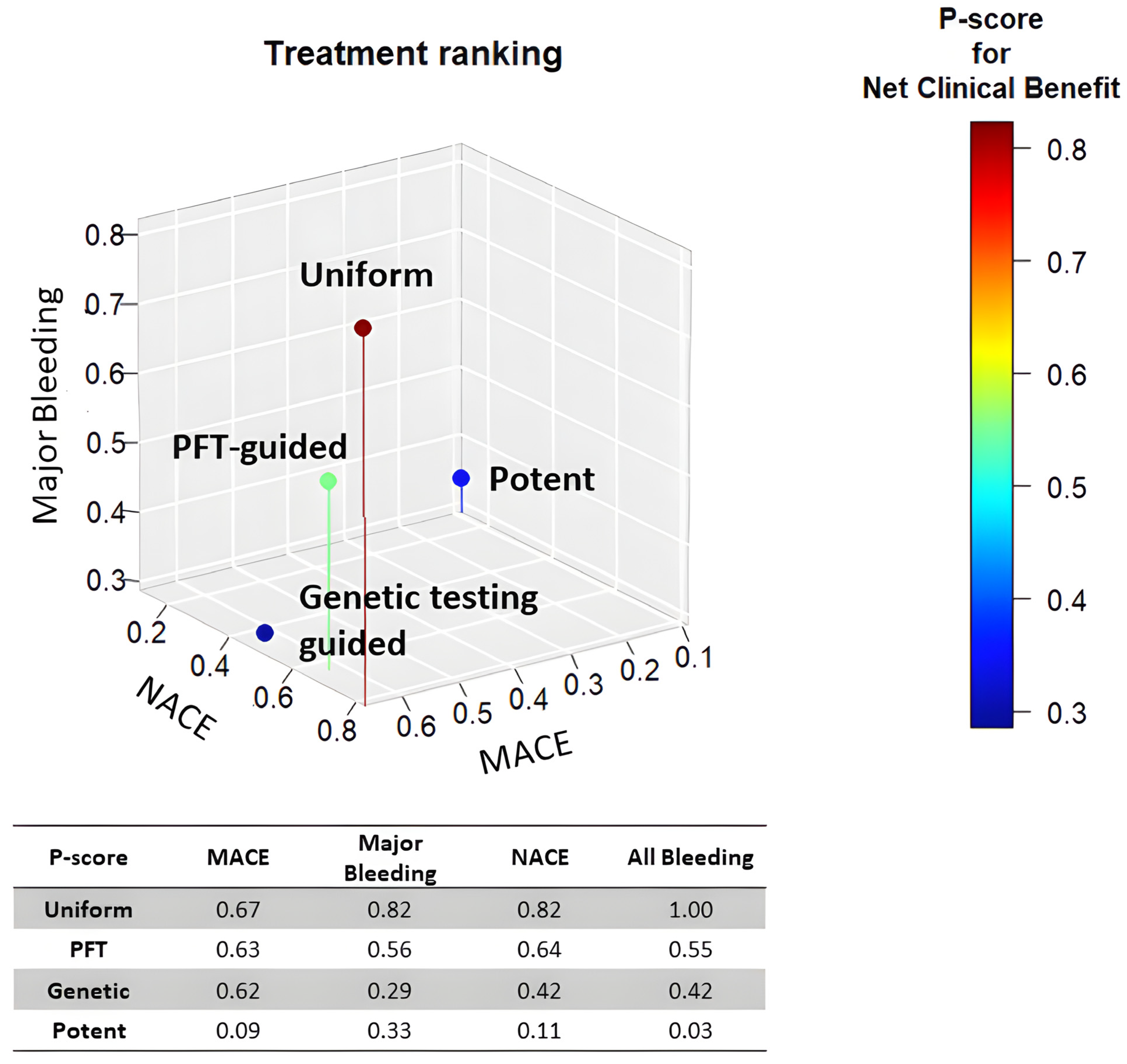
Figure 2. Treatment ranking of P2Y12 de-escalation strategies. The scatterplot depicts the treatment ranking with regards the risk of MACE, Major bleeding, and NACE. Uniform de-escalation was ranked first in all analyses.
In conclusion, uniform unguided P2Y12 de-escalation strategies have consistently shown a reduction in bleeding events without compromising efficacy. Genetic testing-guided de-escalation strategies and de-escalation using PFT guidance provided results showing no difference in bleeding or ischemic events between the de-escalation group and the standard group (4.0% vs. 5.9% and 7% vs. 9%, respectively). Overall, the use of uniform unguided de-escalation appears to be the most effective strategy in reducing bleeding events while maintaining efficacy. However, it is important to note that uniform unguided de-escalation may be associated with an increased risk of ischemic events, that would be more difficult to manage than bleeding, since it does not take into consideration the individual patient's bleeding and ischemic risk in order to select the optimal approach for DAPT abatement, which can lead to serious complications and can be fatal. Further studies will be required to support these assumptions and to determine the most effective approach for individualized patient care.
References
- Lordkipanidzé M. Platelet function tests. Semin Thromb Hemost. 2016;42(3):258-267. doi:10.1055/s-0035-1564834
- Aradi D, Komócsi A, Vorobcsuk A, et al. Prognostic significance of high on-clopidogrel platelet reactivity after percutaneous coronary intervention: systematic review and meta-analysis. Am Heart J. 2010;160(3):543-551. doi:10.1016/j.ahj.2010.06.004
- Harrison P, Lordkipanidzé M. Testing platelet function. Hematol Oncol Clin North Am. 2013;27(3):411-441. doi:10.1016/j.hoc.2013.03.003
- Koltai K, Kesmarky G, Feher G, Tibold A, Toth K. Platelet aggregometry testing: Molecular mechanisms, techniques and clinical implications. Int J Mol Sci. 2017;18(8). doi:10.3390/ijms18081803
- Sibbing D, Aradi D, Alexopoulos D, et al. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y12 Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2019;12(16):1521-1537. doi:10.1016/j.jcin.2019.03.034
- Capodanno D, Baber U, Bhatt DL, et al. P2Y12 inhibitor monotherapy in patients undergoing percutaneous coronary intervention. Nat Rev Cardiol. 2022;19(12):829-844. doi:10.1038/s41569-022-00725-623.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015. doi:10.1056/NEJMoa0706482
- James S, Akerblom A, Cannon CP, et al. Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: Rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J. 2009;157(4):599-605. doi:10.1016/j.ahj.2009.01.003
- Schüpke S, Neumann FJ, Menichelli M, et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2019;381(16):1524-1534. doi:10.1056/NEJMoa1908973
- Price MJ, Angiolillo DJ, Teirstein PS, et al. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial. Circulation. 2011;124(10):1132-1137. doi:10.1161/CIRCULATIONAHA.111.029165
- Collet JP, Hulot JS, Cuisset T, et al. Genetic and platelet function testing of antiplatelet therapy for percutaneous coronary intervention: The ARCTIC-GENE study. Eur J Clin Pharmacol. 2015;71(11):1315-1324. doi:10.1007/s00228-015-1917-9
- Pereira NL, Farkouh ME, So D, et al. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes After Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA. 2020;324(8):761-771. doi:10.1001/jama.2020.12443
- Sibbing D, Aradi D, Jacobshagen C, et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. 2017;390(10104):1747-1757. doi:10.1016/S0140-6736(17)32155-4
- Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: The TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38(41):3070-3078. doi:10.1093/eurheartj/ehx175
- Kim HS, Kang J, Hwang D, et al. Prasugrel-based de-escalation of dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (HOST-REDUCE-POLYTECH-ACS): an open-label, multicentre, non-inferiority randomised trial. Lancet. 2020;396(10257):1079-1089. doi:10.1016/S0140-6736(20)31791-8
- Park MW, Kim CJ, Kim MC, et al. A prospective, multicentre, randomised, open-label trial to compare the efficacy and safety of clopidogrel versus ticagrelor in stabilised patients with acute myocardial infarction after percutaneous coronary intervention: Rationale and design of the TALOS-AMI trial. EuroIntervention. 2021;16(14):1170-1176. doi:10.4244/EIJ-D-20-00187
- Ueno T, Koiwaya H, Sasaki K ichiro, et al. Changes in P2Y12 reaction units after switching treatments from prasugrel to clopidogrel in Japanese patients with acute coronary syndrome followed by elective coronary stenting. Cardiovasc Interv Ther. 2017;32(4):341-350. doi:10.1007/s12928-016-0417-x
- El Alaoui El Abdallaoui O, Tornyos D, Lukács R, Komócsi A. Abatement of potent P2Y12 antagonist-based dual antiplatelet therapy after coronary intervention: A network meta-analysis of randomized controlled trials. Front Cardiovasc Med. 2023;9. doi:10.3389/fcvm.2022.1008914
- Kuno T, Fujisaki T, Shoji S, et al. Comparison of Unguided De-Escalation Versus Guided Selection of Dual Antiplatelet Therapy After Acute Coronary Syndrome: A Systematic Review and Network Meta-Analysis. Circ Cardiovasc Interv. 2022;15(8):e011990. doi:10.1161/CIRCINTERVENTIONS.122.011990
