The current human lifestyle often does not allow for the adoption of healthy diets. Thus, dietary supplementation employing compounds extracted from certain food matrices, as well as new foodstuffs formulated with high concentrations of certain bioactive compounds, may comprise a convenient alternative for human health maintenance.
2. Nitrate
Nitrate (NO
3−) is a negatively charged nitric acid salt formed by a single nitrogen atom bound to three oxygen atoms, while nitrite (NO
2−) is a nitrous acid salt formed by a single nitrogen atom bound to two oxygen atoms. Both can be obtained from endogenous and/or exogenous sources
[42]. Endogenous NO
3− and NO
2− formation occurs by NO metabolism through the L-arginine/NO pathway
[3]. Once in the intracellular medium, the amino acid L-arginine undergoes five-electron oxygen-dependent oxidation catalyzed by the nitric oxide synthase enzyme (NOS) and its cofactors, such as calmodulin, Ca
2+, BH
4, NAD, NADPH, FAD, FMN and O
2, forming NO and L-citrulline
[43]. In addition, shear stress (the blood flow shear force exerted on endothelial cells) can activate NOS to form NO. Once synthesized, NO is rapidly transformed to NO
2− by auto-oxidation or through ceruloplasmin, a protein that plays a role in plasma copper transport. The formed NO
2− can also undergo the action of oxyhemoglobin (oxyHb), generating NO
3− [44].
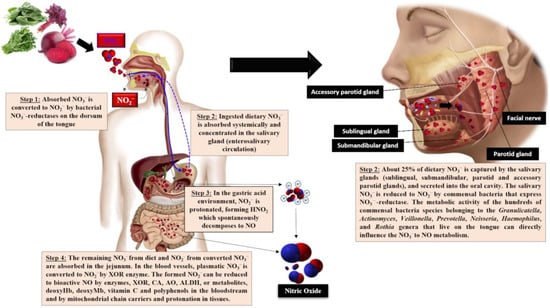
The acquisition of exogenous NO
3− takes place from drinking water and green and leafy vegetables, in addition to vegetables grown in low-light environments, as NO
3− is stored and not reduced to form amino acids. Some tubers, mainly beetroot, store high NO
3− content. In addition, NO
2− is added to cured meat as a preservative additive
[45]. The NO
3− ingested by the NO
3−-NO
2−/NO pathway is absorbed in the proximal portion of the small intestine, possibly the jejunum, into the bloodstream or tissues, where it accumulates intracellularly as NO
3−. Dietary NO
3− increases quickly in plasma in about 30 min, peaking at 90 min. About 60% of the absorbed NO
3− is excreted in urine and 25% is extracted by the salivary glands, concentrated in saliva through the entero-salivary cycle
[46]. Concerning the salivary route, NO
3− in the oral cavity is reduced to NO
2− by nitrate-reductase expressed by oral commensal bacteria, such as
Streptococcus salivarius,
S. mitis,
S. bovis and
Veillonella spp., identified as the most prevalent nitrate-reductive microbiota on the tongue that use NO
3− as a terminal electron acceptor to generate ATP or incorporate it into their biomass
[47][48][49][47,48,49]. This NO
2− mouth generation is sensitive to antibiotics or mouthwash, which can inactivate bacteria, compromising the conversion of NO
3− to NO
2− [50]. Furthermore, the metabolic activities of commensal microorganisms that inhabit the oral cavity, such as
Granulicatella spp.,
Actinomyces,
Prevotella spp.,
Neisseria spp.,
Haemophilus spp. and those belonging to the
Rothia genera, can also significantly influence NO
3− to NO conversion
[51][52][51,52]. Subsequently, NO
2− is protonated upon reaching the gastric acid, forming nitrous acid (HNO
2), which spontaneously decomposes to NO and other bioactive nitrogen oxides, such as nitrogen dioxide (NO
2), dinitrogen trioxide (N
2O
3) and the nitrosonium ion (NO
+). Furthermore, HNO
2 may also be decomposed to NO by ascorbic acid and polyphenols
[48][49][48,49]. In the jejunum, the remaining NO
3− and NO
2− are rapidly absorbed into the bloodstream or tissues. Therefore, NO
2− levels are considerably delayed in circulation, reaching a maximum peak after 2.5–3 h of ingestion
[53], the time required for oral cavity NO
3− to NO
2− conversion (
Figure 1).
Figure 1. Triggering of the NO3−-NO2−/ NO pathway following the ingestion of NO3−-rich foods. XOR, xanthine oxidoreductase; AO, aldehyde oxidase; ALDH, aldehyde dehydrogenase; deoxyHb, deoxyhemoglobin; deoxyMb, deoxymyoglobin; CA, carbonic anhydrase.
Dietary NO
3− and NO
2− accumulation occurs by endogenous synthesis through the L-arginine/NO pathway. As mentioned previously, most NO
3− is lost by renal clearance and a small part is extracted by the salivary glands, concentrating in the saliva, to continue the entero-salivary cycle
[54][55][56][54,55,56]. Additionally, a small amount of plasmatic NO
3− and NO
2− may be reduced by xanthine oxidoreductase (XOR), which displays similar enzymatic activity to salivary nitrate reductase. Xanthine oxidoreductase catalyzes NO synthesis from the remaining NO
3− and NO
2−, albeit in the absence of O
2. Thus, NO can be formed under both hypoxic and ischemic conditions, with increased XOR expression and activity. In addition, NO
2− can be reduced to NO by deoxyhemoglobin (deoxyHb) and deoxymyoglobin (deoxyMb), especially under low O
2 levels
[49][53][49,53]. Other enzymes, such as aldehyde oxidase (AO), aldehyde dehydrogenase (ALDH) and carbonic anhydrase (CA), as well as antioxidant compounds, i.e., vitamin C and polyphenols, display the ability to reduce plasmatic NO
2− to NO, the bioactive form
[54][55][54,55].
As NO
2− is not naturally found in food matrices, due to its instability and quick oxidation to NO
3−, 70 to 80% of NO
2− exposure originates from food additives mixed with foodstuffs. These compounds are used to improve food taste, color and appearance and prevent food oxidation, as well as the growth of foodborne pathogens and secretion of harmful compounds, such as the botulinum toxin, during meat and baked goods and cereal processing
[57]. Thus, plasma NO reflects dietary NO
3− intake, with 85% originating from vegetables in Western diets, although the content of this anion varies between edible plants from distinct botanical families
[50]. Indeed, NO
3− content in vegetables depends on their genetic background or environmental factors such as atmospheric humidity, temperature, water content and exposure to sunlight and irradiation, as well as agricultural practices, i.e., crop type, fertilization, soil conditions, the use of fertilizers and herbicides, the amounts of available nitrogen and the availability of other nutrients, and, finally, post-harvest conditions, such as transportation and storage conditions
[50][58][50,58]. The NO
3− contents in plant organs also differ, classified from the highest to the lowest contents as petiole > leaf > stem > root > tuber > bulb > fruit > seed. Among vegetables considered the richest NO
3− food sources, beetroot (1300 mg of NO
3−·kg
−1), arugula (4677 mg of NO
3−·kg
−1) and spinach (2500 mg of NO
3−·kg
−1) are the most popular with respect to dietary interventions, all resulting in effective cardiovascular performance improvements estimated through blood pressure decreases and vascular function improvements
[49][50][58][49,50,58]. Furthermore, a single serving portion of any of these vegetables contains more NO
3− than is formed through internal human body processes per day. However, it is important to note that NO
3− supplementation from leafy greens has been tested only in healthy individuals, and it is unknown whether its effects can be extended to individuals displaying cardiovascular risk factors. Although the protective cardiovascular effects of NO
3−-enriched vegetables have been clearly demonstrated in clinical trials with healthy subjects, the large vegetable serving portions to be ingested to achieve effective NO
3− plasma concentrations may comprise a limiting factor in ensuring adherence to long-term nutritional interventions
[3]. In this regard, the low NO
3− content in serving portions has been overcome by developing different beetroot formulations that concentrate pharmacological NO
3− doses in small serving portions of an attractive food product, favoring continuous intake and better adherence to a non-drug strategy therapy in order to improve endothelial function in individuals at cardiovascular risk
[3].
However, strict standards regarding the levels of these anions in foods and drinks have been established in the past. Until a decade ago, NO
3− was considered a toxic compound derived from unfavorable diets, as it was mistakenly associated with the development of certain malignancies, such as metglobinemia (MetHba) and gastric cancer
[59][60][61][59,60,61]. Therefore, the Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) defined an acceptable daily intake of 3.7 mg of NO
3−·kg
−1 of body weight in 1962, the same level adopted by the European Food Safety Authority
[62][63][62,63]. For a healthy 80 kg adult, this content is the equivalent of ~300 mg NO
3−·day
−1. However, the adoption of vegetarian diets, in general, increases NO
3− consumption in 80 kg adults to over 350 mg.day
−1, well above the stipulated acceptable daily intake
[64]. The association between NO
3− and NO
2− and MetHba in adults and children, however, has not been proven in the literature
[61][65][61,65]. Furthermore, several studies have failed to demonstrate a link between dietary NO
3− and NO
2− ingestion and the production of N-nitrosamines, carcinogenic compounds that can lead to tumor development
[66]. This evidence supports a significant link between cancer and red processed meat, with little knowledge of the effects of vegetables and drinking water available. In this regard, the inorganic NO
3− and, particularly, inorganic NO
2− added during meat processing may contribute to cancer development
[67]. Nonetheless, the hypothesis that both dietary NO
3− and NO
2− from foods, mainly from plant origin, are toxic has been established based merely on conjecture.
Health organizations have established an adequate NO
3− intake of around 40–185 mg·day
−1 (1 to 3 mmol·day
−1) in Europe and 40–100 mg·day
−1 (1 to 1.6 mmol·day
−1) in the USA, considering 100% NO
3− bioavailability following dietary intake
[68]. However, considering the role of NO
3− on cardiovascular system function, none or minimal beneficial hemodynamic and vascular effects have been observed following acute NO
3− administration or short-period administration for under 14 days
[3][58][3,58]. Increases in plasmatic NO
3− levels, from 31 to 150 μM, as well as NO
2−, from 0.23 to 0.40 μM, have been observed, but no improvements were detected in SBP and FMD following 14 days of supplementation with 7.5 mmol NO
3− from beetroot juice in elderly patients with type 2 diabetes mellitus
[69]. No changes in arterial stiffness, assessed by PWV and AIx, or in blood pressure were observed in normotensive individuals after a 7-day intake of 6.4 mmol NO
3− from green leafy vegetables, although increased plasmatic NO
3− levels, from 23.4 to 152 μM, and NO
2−, from 2.0 to 8.0 μM, were observed
[70]. Furthermore, Bondonno et al.
[71] did not observe modifications in domestic BP, and ambulatory 24 h SBP and DBP in hypertensive individuals supplemented for 7 days with 7.0 mmol NO
3− from beetroot juice, although increased NO synthesis was observed, assessed through NO
3− and NO
2− determinations in plasma, urine, and saliva.
On the other hand, when NO
3− is provided as a chronic dietary supplementation, the beneficial effects on vascular function are more consistents. Kapil et al.
[72] observed a decrease in systolic (SBP) and diastolic blood pressure (DBP), decrease in PWV, and an increase and improvement of AIx and FMD, respectively, of hypertensive volunteers after supplementation with 6.4 mmol NO
3− during 28 days, corresponding to 400 mg/day of beetroot juice. Endothelial function and arterial stiffness improvements and decreased blood pressure were observed simultaneously with increased NO synthesis, estimated by increased NO
3− plasma levels, from ≈40 to ≈200 μM, as well as higher NO
2− levels, from ≈0.4 to ≈0.9 µM. Rammos et al.
[73] administered 150 µmol·kg
−1 of NaNO
3−, at least 10.5 mmol for a 70 kg individual, for 28 days to elderly patients presenting moderate CVD risks, also describing increases in average NO
3− plasma concentrations, varying from 32 to 263 μM, and NO
2− concentrations, ranging from 0.08 to 0.33 μM, alongside improved FMD and decreased SBP, DBP, PWV and AIx. Recent systematic reviews and meta-analysis studies have evaluated vascular responses to dietary NO
3− and confirmed BP decreases and endothelial dysfunction amelioration. A systematic review conducted by Bahadoran et al.
[74] demonstrated that beetroot juice intake reduces SBP [−3.55 mm Hg; 95% CI: −4.55, −2.54 mm Hg] and DBP [−1.32 mm Hg; 95% CI: −1.97, −0.68 mm Hg]. Furthermore, decreased SBP depends on the amount of beetroot juice intake, where comparisons between 500 mL and 70 mL and 140 mL·day
−1 indicate −4.78 vs. −2.37 mm Hg decreases. Longer supplementation periods compared to shorter ones (≥14 days vs. <14 days of treatment) led to a −5.11 vs. −2.67 mm Hg decrease. In another systematic review and meta-analysis, randomized controlled trials indicated that NO
3− supplementation from beetroot juice for longer than 14 days reduced both SBP (−3.55 mm Hg; 95% CI: −4.55, −2.54 mm Hg) and DBP (−1.32 mm Hg; 95% CI: −1.97, −0.68 mm Hg). Furthermore, beneficial dietary NO
3− effects on endothelial function are associated with dose, age, and body mass index (BMI), where chronic beet juice supplementation improved FMD and endothelium function according to the administered NO
3− dose (β = 0.04, SE = 0.01,
p < 0.001), age (β = −0.01, SE = 0.004,
p = 0.02) and BMI (β = −0.04, SE = 0.02,
p = 0.05)
[75].
3. L-Arginine
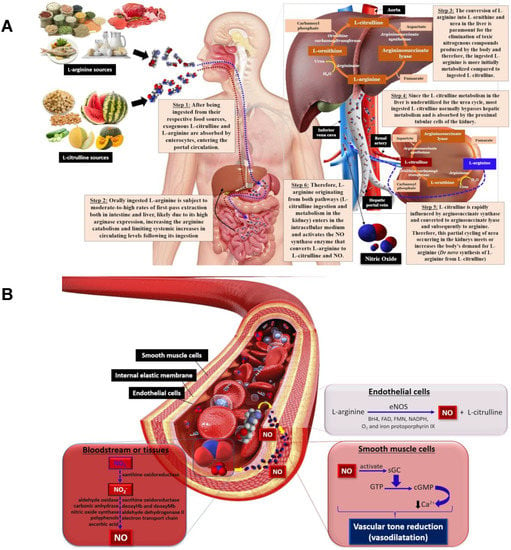
L-arginine (2-amino-5-guanidinopentanoic acid) is a semi-essential cationic amino acid obtained through dietary intake, protein turnover, and/or de novo synthesis from L-citrulline in liver, and from the kidney urea cycle
[76][90]. Oral L-arginine undergoes gastrointestinal and hepatic extractions before reaching portal circulation, where arginases from enterocytes and liver catalyze the hydrolysis of L-arginine into L-ornithine and urea, which limits systemic L-arginine levels (
Figure 2A). L-arginine is also biosynthesized in the kidneys through L-citrulline metabolism following the conversion of exogenous L-citrulline (not metabolized in the liver first-pass) to the precursor arginosuccinate, catalyzed by arginosuccinate synthase, and then converted to L-arginine by arginosuccinate lyase in the urea cycle
[76][90].
Figure 2. The metabolic pathway for L-arginine and L-citrulline obtained from food sources, urea cycle and NO biosynthesis (A); NO biosynthesis from L-arginine by eNOS promotes vasodilation by activating guanylate cyclase to form cGMP that, in turn, decreases Ca2+ within smooth muscle cells, diminishing vascular tone and leading to vasodilation (B). eNOS—endothelial nitric oxide synthase, BH4—tetrahydrobiopterin, FAD—flavin adenine dinucleotide, FMN—flavin mononucleotide, GC—guanylate cyclase, GTP—guanosine triphosphate, cGMP—cyclic guanosine monophosphate, Ca2+—calcium ions.
L-arginine is involved in NO synthesis, and is employed as a substrate for nitric oxide synthase (NOS) class enzymes, comprising neuronal (nNOS), inducible (iNOS) and endothelial (eNOS) isoforms. As mentioned previously, L-arginine, the substrate, adenine dinucleotide phosphate (NADPH) as the electron cofactor, and O
2 are involved in NO synthesis, forming citrulline and NADP
+. Tetrahydrobiopterin (BH
4), flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN) and iron protoporphyrin IX are all cofactors involved in this reaction
[77][91]. Once synthesized, NO diffuses from endothelial cells to smooth muscle cells in blood vessels and activates the soluble guanylate cyclase (GC) enzyme that, in turn, catalyzes the conversion of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP) and pyrophosphate (PPi)
[78][92]. Subsequently, cGMP decreases intracellular Ca
2+ concentrations by activating the calcium pump within smooth muscle cells, inducing vasodilation through reduced vascular tone
[79][80][81][93,94,95] (
Figure 2B).
L-arginine is found in nuts, such as peanuts and walnuts, but also in foods of animal origin, such as meats, poultry, fish and dairy products, providing an intake of about 4.4 g/day of this amino acid in Western diets
[82][96]. Considering that L-arginine is the NO substrate for NO synthesis and its involvement in endothelium-dependent dilatation, the beneficial effects of L-arginine supplementation on endothelial dysfunction and arterial stiffness have attracted attention for some time, aiming to overcome hemodynamic abnormalities and risk factors, thus avoiding cardiovascular events.
In a prospective, double-blind, randomized crossover trial with elderly healthy individuals (age 73.8 ± 2.7 years), the L-arginine supplementation of 16 g/day for 2 weeks increased L-arginine plasma levels from 57.4 ± 5.0 mM to 114.9 ± 11.6 mM, improving endothelial-dependent vasodilation
[34]. Similar to aging, smoking represents a harmful condition leading to endothelial dysfunction and decreased NO biosynthesis alongside inflammatory responses caused by endothelium injury, which can be counteracted by L-arginine supplementation. Six grams/day of L-arginine administered for 3 days to 10 healthy smokers in a randomized, placebo-controlled, double-blind, and cross-over clinical trial led to an improvement on FMD baseline and prevented smoking-induced FMD decreases at day 1. The supplementation was, however, unable to sustain this up to day 3. On the other hand, L-arginine decreased both PWV and AIx at days 1 and 3 compared to the placebo
[83][97]. In a similar study, L-arginine supplemented at 21 g/day for 3 days to 12 healthy smokers improved FMD baselines, but did not cease smoking effects, while smoking-induced PWV and AIx increases were inhibited by L-arginine supplementation
[84][98]. L-arginine may, therefore, be considered a very promissory positive effector, able to prevent the arterial stiffness increment associated with smoking behavior. L-arginine also seems to improve endothelial function in subjects presenting coronary artery disease (CAD)
[85][99]. Following an intake of 21 g of L-arginine for 10 days resulted in an FMD improvement by 4.7 ± 1.1 vs. 1.8 ± 0.7% (
p < 0.04). Similarly, the intake of 10 g of L-arginine by stable CAD patients for a longer period of time of 4 weeks reduced endothelial dysfunction, as demonstrated by changes in FMD diameter, increasing the diameter by 4.87% (
p < 0.0001) compared to baseline values. The conclusions from that study, however, were limited, due to its open-label clinical trial character, as the placebo group received vitamin C, which is also an active compound
[86][100]. On the other hand, in a similar clinical double-blind and placebo-controlled trial, chronic L-arginine therapy was shown to increase the reactive hyperemia of the forearm blood flow by employing venous occlusion plethysmography in individuals displaying stable CAD for 6 months. The hyperemic flow of the forearm is considered an endothelial-mediated vasodilation marker and has been shown to depend on NO synthesis. The positive correlation between the 6.4 g/day L-arginine supplementation, c-GMP and reactive hyperemia increments reinforces the role of L-arginine in NO synthesis enhancement and consequent endothelial function improvement
[87][101]. Coronary endothelial dysfunction has been proposed to predict the progression of atherosclerotic disease and cardiovascular event rates
[88][102], and, although the clinical trials presented in this re
svie
archw have not evaluated the effect of L-arginine on the coronary artery, the findings presented herein indicate that L-arginine supplementation may comprise a promising alternative against endothelial function impairment in CAD patients and be able to inhibit unfavorable cardiovascular outcomes.
L-arginine intake for 28 days or more has also been shown to protect against others chronic pathologies deleterious to vascular function. The daily ingestion of 8 g L-arginine improved endothelial function in 28 women with polycystic ovary syndrome (PCOS) who were making use of oral contraceptives. PCOS is a pathological condition associated with low NO bioavailability due to its inadequate release, production, or degradation. This results in endothelium-dependent vasodilatation impairment and cardiovascular risks that may be exacerbated by oral contraceptives
[89][90][103,104]. For the treatment of PCOS, ultrasonographic and Doppler flow evaluations revealed that L-arginine supplementation promoted a significant improvement in brachial artery diameter and pulsatility index at 15 s after reactive hyperemia, compared to the baseline. This effect was not observed in contraceptive plus placebo group. L-arginine supplementation did not lead to any blood pressure variations, whereas the placebo group displayed increases for 24 h, both during the day and during the night. Increased plasma NO
3− and NO
2− following L-arginine intake confirmed NO availability and endothelial function improvements. However, the most important finding in this intervention comprised the fact that endothelial function and NO production effects were extended for 6 months following L-arginine supplementation, demonstrating a sustained effect following 28 days of L-arginine administration
[90][104].
Oral administration of 8 g L-arginine to 15 congestive heart failure patients for 60 days led to endothelial function improvements, evaluated through the maximum amplitude time (MAT), total wave time (TT) and MAT/TT ratio obtained through photoplethysmography following forearm blood flow occlusion and reactive hyperemia in comparison to pre-ischemia levels
[91][105]. Photoplethysmography can be used to indirectly evaluate endothelial function in peripheral vessels by sensing vasodilatation in the index finger, since changes in blood flow and pulse wave amplitude are the result of flow-mediated vasodilatation following NO synthesis
[92][106]. Although the dietary intervention was limited due to the low number of patients, the absence of a control group, and the method reflecting only the microvascular status, L-arginine supplementation decreased MAT/TT values to under 30, similar to values observed in healthy individuals, demonstrating its modulation on NO-mediated vasodilation
[93][107]. Likewise, a 12-week supplementation with 9 g of L-arginine was able to circumvent the PWV ≥ 900 cm/s commonly found in chronic kidney disease (CKD) patients. Clinical CKD manifestations increase the risks for CAD, heart failure and cardiac death due to the pathological vascular remodeling, calcification and arterial stiffness that underly kidney disease
[93][107]. The chronic intake of L-arginine promoted a significant increase in NO levels and decreased aortic stiffness, overcoming NO baseline levels and PWV, confirming vascular damage caused by kidney impairment and the effectiveness of L-arginine supplementation in reversing hemodynamic abnormalities in CKD patients, probably caused by a defective L-arginine/NO biosynthesis
[94][108].
Postprandial endothelial dysfunction is a clinically important condition and has been reported following high-fat meals in both healthy subjects and in those with risk factors for CVD. Postprandial endothelial dysfunction has been proposed to be triggered through oxidative stress induced by hypertriglyceridemia, where NO bioavailability is reduced by the superoxide anion (O
2−), resulting in the generation of the highly reactive and cytotoxic peroxynitrite
[95][96][97][109,110,111]. In overweight adults with high triglyceride plasma levels and waist circumference measurements, L-arginine supplementation was able to overcome cardiovascular risk factors following 4.5 g supplementation for 4 weeks, where an inhibition of the decrease in postprandial endothelial function induced by a high-fat meal was observed, demonstrated by a 29% reduction in FMD when compared to a 50% reduction in FMD in placebo-treated subjects. In this clinical trial, a 5% increase in reactive hyperemia was also observed, while there was a 49% reduction in placebo treatment
[98][112]. Similarly, a single dose of 15 g of L-arginine attenuated the FMD reduction promoted by high-fat meals compared with a placebo treatment in forty healthy men (from 10.3 ± 1.3 to 9.3 ± 0.9% in the L-arginine supplementation group and from 10.5 ± 1.2% to 6.8 ± 1.4%, in the placebo group)
[99][113]. These findings indicate that L-arginine may increase NO bioavailability and reduce endothelial dysfunction induced by postprandial hypertriglyceridemia.
4. L-Citrulline
L-citrulline, a non-essential amino acid not commonly found in proteins, is involved in nitrogen homeostasis and shows promising vascular benefits in promoting endothelial vasodilation. L-citrulline is efficiently converted to L-arginine, the endogenous NO biosynthesis precursor that acts in reducing arterial stiffness, as discussed previously (
Figure 2B)
[35][100][101][35,116,117]. Dietary L-citrulline passes through the liver to the kidneys, where it is converted to L-arginine via the argininosuccinate biosynthesis in the urea cycle, producing extra NO (
Figure 2A). Studies have reported that L-citrulline can indirectly reduce blood pressure by increasing NO biosynthesis, improving arterial function, blood flow and circulation and, thus, reducing the risk of heart disease
[102][103][118,119].
L-citrulline is found in legumes, fruits, and grains, such as onions, garlic, chickpeas, peanuts, and soy, and the highest concentrations of this amino acid are found in watermelon pulp and rinds in concentrations ranging between 0.7 and 3.6 g.kg
−1 of fresh weight depending on the type of cultivar
[101][104][105][117,120,121]. L-citrulline has been widely commercialized as a supplement, and is available at higher doses than those found in natural foods. Marketed L-citrulline is mainly consumed by athletes to assist in sports performance and muscle mass gain
[106][107][122,123].
Although L-citrulline was for many years considered simply a metabolic intermediate of little biological interest, studies have confirmed that it increases circulating L-arginine levels more efficiently than L-arginine supplementation, the final product formed during L-citrulline metabolization. A large amount of the ingested L-arginine is degraded during the extensive pre-systemic metabolism by intestinal bacteria and by arginase found in the liver and gut mucosa. In contrast, L-citrulline is preserved during the pre-systemic metabolism, effectively transported across the intestinal luminal membrane and then converted to L-arginine in the kidneys. Subsequently, L-arginine is converted to L-citrulline and NO by eNOS in endothelial cells
[35][54][76][103][108][35,54,90,119,124]. The absorption characteristics of oral L-citrulline indicate that the use of this amino acid comprises an attractive non-pharmacological approach that may counteract cardiovascular pathophysiological conditions.
The endothelial function effects of watermelon ingestion for seven consecutive days were investigated on the basis of FMD measurements. Six healthy overweight/obese adults received 100 kcal serving portions prepared from watermelon pulp, rind, and seeds, while the control group received flour. FMD reactivity assessed in the brachial artery after 7 h intake showed no differences, with the authors ascribing the inconclusive results to a low sample number
[109][125]. When evaluating the supplementation of eleven young adults with 30 g of microencapsulated watermelon rind (MWR) containing 4 g of L-citrulline, improved endothelial function assessed by FMD was observed alongside with increased L-citrulline and L-arginine plasma levels
[110][126].
One hypothesis postulate that postprandial hyperglycemia and acute hyperlipidemia both induce endothelial dysfunction as measured by FMD throughout oxidative stress induction, since free radicals quench NO, disrupting endothelial-dependent vasodilation
[96][110]. In a randomized, placebo-controlled, double-blind, crossover trial, 17 healthy young adults from 21 to 25 years old, 6 males and 11 females, were supplemented with 500 mL of watermelon juice for 2 weeks and underwent an oral glucose tolerance test followed by postprandial FMD, to evaluate endothelial function following hyperglycemia induction and L-citrulline supplementation effects. Although no significant effects were observed on plasma L-citrulline and L-arginine, the latter showed a tendency to increase compared to placebo and the postprandial FMD area AUC was higher after juice supplementation when compared with the placebo group (838 ± 459% vs. 90 min compared with 539 ± 278% vs. 90 min).
[111][127]. In this way, supplementation with L-citrulline, as a precursor of L-arginine and consequently of nitric oxide, seems to have the potential to attenuate the endothelial dysfunction induced by high glucose levels, but more studies need to be carried out.
Another randomized, double-blind, placebo-controlled trial study was performed to evaluate the effects of watermelon juice on vascular health, albeit in 21 healthy postmenopausal women. Subjects were randomized to consume two 360 mL servings of 100% watermelon juice ingested daily or an isocaloric placebo for 4 weeks. Vascular function assessments included pulse pressure, PWV, 24 h ambulatory BP, and FMD. In contrast to the findings of previous clinical trials in younger adults, the watermelon juice supplementation did not affect vascular parameters compared to the placebo, indicating that a 720 mL dosage/day of watermelon juice is insufficient to alter serum L-arginine in postmenopausal women, possibly explaining the unchanged vascular function
[112][128]. On the other hand, watermelon supplementation (L-citrulline/L-arginine 6 g/d) to twelve obese, hypertensive postmenopausal women for 6 weeks significantly lowered baPWV, aortic SBP and DBP compared to placebo
[113][129], which suggests that the vascular effects of watermelon L-citrulline may be more pronounced when the individual has some cardiovascular risk factor.
Following randomized crossover studies, watermelon intake enriched in L-citrulline promoted aortic blood pressure and arterial function amelioration in hypertensive individuals. The pilot study assessed nine participants, four men and five women aged 54 ± 3 years, who were diagnosed with pre-hypertension and consumed 2.7 g/day of watermelon or placebo for 6 weeks. Both the AIx and AIx adjusted for an HR of 75 beats/min (AIx 75) decreased in the watermelon-supplemented group (−6.0 ± 3% and −4.0 ± 2%, respectively). At the same time, no PWV carotid–femoral or reflected wave (T
r) transit time effects were noted
[114][130]. In another study, carotid AIx (cAIx) measures were performed instead of aortic AIx, since the former can more precisely reflect the central AIx. After 6 weeks of watermelon supplementation containing 6 g of L-citrulline/L-arginine administered daily, decreased cAlx values (−8.8 ± 2.6%) were observed in 14 obese middle-aged adults presenting prehypertension or stage 1 hypertension, reflecting artery endothelium function improvements
[103][119].
Acute ingestion of L-citrulline (3 g) effectively increases the availability of L-arginine and NO in both young and elderly adults with heart failure. L-citrulline supplementation increased NO synthesis 10-fold, but was ineffective at promoting endothelium-mediated vasodilation in the two CVD subject groups. These results imply that other factors besides NO may play a role in vascular dysfunction. In addition, longer-term L-arginine or L-citrulline supplementation is required to reverse peripheral vascular function impairment in older adults with heart failure. Younger adults are more sensitive to the acute ingestion of L-citrulline compared to the elderly, due to the efficacy in converting this compound to L-arginine. Older adults present a higher L-arginine to ornithine conversion rate via arginase in the urea cycle, illustrating NO synthesis differenced with aging
[115][131].