Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Magdalena Oroń and Version 2 by Rita Xu.
Alternative pre-mRNA splicing is a process that allows for the generation of an extremely diverse proteome from a much smaller number of genes. In this process, non-coding introns are excised from primary mRNA and coding exons are joined together. Different combinations of exons give rise to alternative versions of a protein.
- alternative splicing
- mutant p53
- KRAS
- CMYC
- splicing factors
1. Introduction
Alternative pre-mRNA splicing is a common process in higher eukaryotes that allows for the generation of an extremely diverse proteome from a much smaller number of genes [1][2][1,2]. It is estimated that 95% of human multiexon genes undergo alternative splicing [3]. Alternative splicing plays a crucial role in development and tissue differentiation by providing proteins with different functions from the same set of genes [4]. Disruption of this process may result in disturbed cell differentiation and oncogenesis. The pattern of alternative exon selection in cancer resembles the one in corresponding embryonic tissues and involves the same regulatory pathways [5]. Multiple mRNA-Seq datasets comparing tumors and corresponding normal tissues demonstrate that an aberrant splicing landscape is a common feature of cancer [6][7][8][6,7,8]. Alternative oncogenic isoforms of different proteins are involved in all hallmarks of cancer: growth signal independence, avoidance of apoptosis, unlimited proliferation, genome instability, motility and invasiveness, angiogenesis, and metabolism [9][10][9,10].
mRNA splicing involves the spliceosome (a large ribonucleoprotein complex composed of snRNAs and associated proteins) and splicing regulatory factors [11]. In hematological malignancies, point mutations in spliceosome components (e.g., SF3B1, SF3A1, U2AF1, or ZRSR2) are more frequent, while in solid tumors, splicing is deregulated mostly by upregulation or downregulation of splicing regulatory factors (e.g., SRSF1, TRA2β, hnRNPA1, or QKI) [12]. Splicing is tightly regulated at various levels: by linking with transcription rate [13][14][13,14], expression, nonsense-mediated decay, and post-translational modifications of splicing factors [15][16][17][15,16,17]. In addition, relative levels of snRNAs U1, U2, U4, U5, and U6—basic components of the spliceosome—may also be important [18]. All of these can be disrupted by the activation of proto-oncogenes.
Altered splicing may also increase the activity of driver oncogenes and oncogenic signaling pathways via preferential expression of alternative pro-oncogenic isoforms, creation of new splice sites leading to the expression of cancer-specific isoforms, or indirectly via changed splicing of regulatory proteins. Specific examples will be discussed in the following paragraphs.
2. Alternative Splicing under the Control of Driver Oncogenes
2.1. CMYC
The oncogenic activity of CMYC in different cancers has been extensively studied and well documented. This proto-oncogene belongs to the family of transcription factors, along with NMYC and LMYC [19][23]. In normal tissues, its level is low and tightly regulated on the transcriptional and post-transcriptional level. In cancers, CMYC level is frequently increased via various mechanisms: gene amplification or translocation, upregulation of signaling pathways boosting CMYC activity, post-translational modifications, and increased protein stability [20][21][24,25]. Activated CMYC is a hallmark of many cancers and is required for their initiation and maintenance [22][26]. The transcriptional program of CMYC and its intersections with other oncogenes have been broadly discussed elsewhere [23][24][27,28]. Here, rwesearchers will focus only on the role of CMYC in the regulation of alternative splicing and its consequences for oncogenesis. The globally increased transcription induced by CMYC requires adaptation of the splicing and translation machinery. The shortage of spliceosome components and splicing regulatory factors affects splicing profile [25][26][29,30]. CMYC hyperactivation increases translation of a core spliceosome component, SF3A3, through an eIF3D-dependent mechanism. This affects the splicing of many mRNAs, including those involved in mitochondrial metabolism favoring stem-cell-like, cancer-associated changes [27][31]. Koh et al. demonstrated that CMYC directly upregulates the transcription of several small nuclear ribonucleoprotein particle assembly genes, including PRMT5, an arginine methyltransferase. This protein methylates Sm proteins that form the Sm core of the spliceosomal snRNPs U1, U2, U4/U6, and U5, and is critical for correct spliceosome assembly [28][32]. The perturbation in PRMT5 expression caused by CMYC leads mainly to intron retention or exon skipping and disturbs splicing fidelity [26][30]. CMYC not only causes overexpression of the most studied oncogenic splicing factor, SRSF1 [29][33], but also causes overexpression of several other proteins from the same family. Urbanski et al. identified an alternative splicing (AS) signature associated with high CMYC activity in breast cancer and showed that the change in AS is caused by the co-expression of splicing factors modules. They also demonstrated that overexpression of at least one module of the splicing factors SRSF2, SRSF3, and SRSF7 correlated with high CMYC activity across thirty-three cancer types [30][34]. The transcriptional activity of CMYC indirectly affects other oncogenes and key oncogenic processes by deregulating splicing. Through the overexpression of hnRNPH splicing factor, CMYC affects the RAS/RAF/ERK signaling pathway. A high level of CMYC and hnRNPH correlates with the expression of full-length A-RAF kinase. A-RAF activates oncogenic RAS signaling and inhibits apoptosis by binding to proapoptotic MST2 kinase. In cells with a low level of CMYC and hnRNPH, A-RAF is spliced into a shorter isoform that cannot bind to MST2. A-RAFshort still binds to RAS, but in a way that inhibits RAS activation [31][35]. One of the hallmarks of cancer is the conversion of glucose into energy via aerobic glycolysis instead of oxidative phosphorylation. This process is governed by PKM2, a pyruvate kinase isoform expressed mainly in embryonic and cancer cells, in contrast to the PKM1 isoform present in most adult normal cells. Overactive CMYC upregulates transcription of PTBP1, hnRNPA1, and hnRNPA2 splicing factors, resulting in a high PKM2/PKM1 ratio [32][36]. Zhang et al. found that another member of the MYC family, NMYC, similarly causes the upregulation of the same splicing factors, PTBP1 and HNRNPA1, leading to the expression of pro-oncogenic PMK2 isoform [33][37]. CMYC not only initiates transcription but also regulates transcription rate [34][38]. A pre-mRNA splicing occurs co-transcriptionally and the transcription rate influences the selection of alternative exons [13][35][13,39]. CMYC controls the transcription and splicing of a splicing factor, Sam68 pre-mRNA, in prostate cancer by binding to the promoter and increasing the RNA Pol II processivity [36][40]. Splicing factors (SF) frequently use an autoregulatory loop to control their own level: when SF protein level is high, it induces incorporation of a poison exon that triggers nonsense-mediated decay (NMD) of the transcript. In prostate and breast cancer, it was shown that CMYC not only increased the transcription of SRSF3 (and several other splicing factors) but also prevented incorporation of the poison exon [37][41]. The exact mechanism remains to be elucidated, but one may hypothesize that the exon skipping is caused by the increased transcription rate induced by CMYC. Selected effects of activated CMYC on splicing are summarized in Figure 1.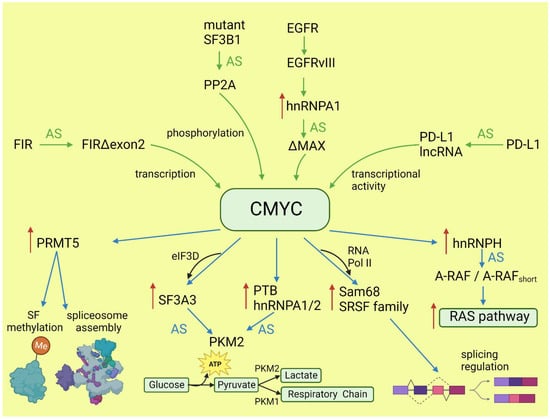
Figure 1. Effects of CMYC on splicing (blue arrows) and the influence of altered splicing on CMYC (green arrows). Red arrows: increased expression; AS: alternative splicing; SF: splicing factor.
2.2. RAS and Downstream Pathways
KRAS, together with NRAS and HRAS, belongs to the small GTPases family, which under physiological conditions cycles between active (GTP-bound) and inactive (GDP-bound) conformations in response to stimulation of cell surface receptors such as HER2, EGFR, or CMET. KRAS is commonly mutated in human cancers; however, the percentage of mutations varies between cancer types [38][42]. Mutations most frequently occur in codons for glycine at amino acid position 12 or 13, causing KRAS hyperactivity that contributes to its transforming properties [39][43]. Signaling cascades are triggered by the binding of the active RAS to RAS-binding domains within several known RAS effector pathways. The most important, in the cancer context, are the PI3K-AKT and MAPK signaling pathways [40][44]. Lo et al. found that in lung cancer cells expressing either WT or mutant KRAS variants, the phosphorylation of SR splicing factors was reduced in those with mutant KRAS expression. This observation correlated with changed cassette exon skipping or inclusion in mutant vs. WT KRAS [41][45]. Correct phosphorylation of SR proteins by SRPK1 and CLK1 kinases is indispensable for their binding to splicing enhancer sequences on pre-mRNA [42][46]. It is known that signaling through PI3K/AKT activates SRPK1 kinase [43][47]; however, the link between RAS and SRPK1 activation was not directly demonstrated. In hepatocellular carcinoma, activation of the RAS/PI3K/AKT pathway leads to the expression of KLF6 splicing variant 1 (KLF6-SV1), which antagonizes the function of full-length KLF6 [44][48]. This protein is a tumor suppressor. The sorter isoform is an oncogene found in many tumors and involved in proliferation, metastasis, and angiogenesis [45][49]. KLF6 splicing is regulated by SRSF1 splicing factor [46][50]. Cheng et al. found a positive feedback loop involving activated RAS and an alternative isoform of CD44 (CD44v6) that can be a coreceptor of growth factor receptors. Activation of the RAS/MAPK/ERK pathway correlated with a preference for exon v6 inclusion. CD44v6, in turn, sustained signaling through tyrosine receptor kinases which activate RAS and downstream pathways, boosting cancer cell proliferation. The author suggested that this positive feedback loop could be a mechanism of the RAS-dependent pathway’s activation in cancers without oncogenic RAS mutations [47][51]. Alternative splicing of CD44 is regulated by a splicing factor, Sam68, which is phosphorylated by ERK, an effector of the RAS/MAPK/ERK pathway [48][52]. The same signaling pathway may be responsible for alternative pro-oncogenic splicing of other Sam68-dependent genes, namely, CCND1, SRSF1, BCL-xL, and mTOR [49][53]. Notably, mTOR is a key effector part of the PI3K/AKT/mTOR pathway that can be activated by RAS [40][44]. The effects of mutated KRAS on splicing are summarized in Figure 2.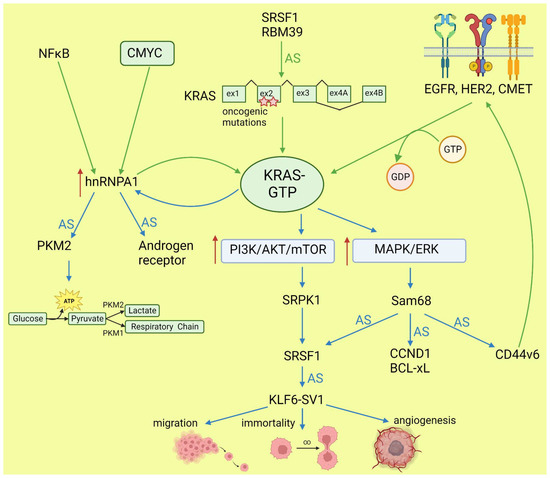
Figure 2. Effects of KRAS on splicing (blue arrows) and influence of altered splicing on KRAS (green arrows). AS: alternative splicing; red arrows: increased expression or pathway activity.
2.3. Mutant p53
The TP53 gene is a tumor suppressor, while its hotspot mutants gain oncogenic activity (named gain of function, GOF) and are involved in all hallmarks of cancer [50][54]. In contrast to wild-type proteins, p53 GOF mutants do not bind directly to promoters, but interact with different transcription factors to control expression of target genes [51][55]. In addition to transcription control, p53 mutants contribute to oncogenesis via interaction with TAp63 and TAp73 [52][56] to inhibit their proapoptotic activity, and with ID4 in angiogenesis stimulation [53][54][57,58]. While many researchers have studied the alternative splicing of the TP53 transcript and demonstrated the role of alternative isoforms in cancer development (discussed in more detail later), little is known about the role of mutant p53 in alternative splicing regulation. Escobar-Hoyos et al. demonstrated that in pancreatic cancer, mutant p53 upregulates the expression of hnRNPK splicing factor. This leads to the mis-splicing of GAP proteins and activates the oncogenic RAS pathway [55][59]. In addition, the RNA-Seq results underlying this study indicate other genes alternatively spliced under the influence of the p53 mutant. Pruszko et al. found, in breast cancer, a ribonucleoprotein complex composed of mutant p53, a splicing factor SRSF1, ID4, and lncRNA MALAT1. The complex altered a splicing of the VEGFA transcript, thus promoting proangiogenic isoforms over antiangiogenic isoforms [54][58]. A proangiogenic role of the studied complex was confirmed in vivo in a zebrafish model [56][60]. These two independent studies indicated that GOF p53 mutants influence alternative splicing in cancer. However, published results are limited to two cancer types and focused on a few target genes. Further studies based on a broad spectrum of tumors are needed to understand the role and interactions with other oncogenes of the GOF p53 mutants in alternative splicing regulation.3. Driver Oncogenes Regulated by Alternative Splicing
3.1. TP53 Regulation by Splicing
The TP53 gene produces at least 12 isoforms with different features that might prevent or promote cancer development. The canonical, full-length p53 (FLp53) protein has seven functional domains: N-terminal transactivation domains TAD1 and TAD2, a proline-rich domain, a DNA-binding domain, a nuclear localization signal, an oligomerization domain, and a negative-regulation domain [57][61]. The α (full-length), β, and γ variants of p53 result from alternative splicing of the C-terminus. β and γ isoforms are formed by partial retention of intron 9 (i9). The resulting alternative exons, 9b or 9g, contain a stop codon, leading to the replacement of the oligomerization domain into 10 amino acids (DQTSFQKENC) in p53β and 15 amino acids (MLLDLRWCYFLINSS) in p53γ (Figure 3). SRSF1 and SRSF3 regulate the splicing of i9 and favor the expression of FLp53 [58][59][62,63]. Marcel et al. observed that inhibiting CLK kinase or silencing SRSF1 upregulated the expression of p53β and p53γ isoforms in the breast cancer cell line MCF7 [59][63]. In contrast to SRSF1 and SRSF3, SRSF7 has been reported to enhance p53β expression in response to ionizing radiation [60][64]. Each of the α, β, and γ isoforms may also be changed from the N-terminus through alternative splicing of intron 2 (Δ40), alternative start of translation from internal IRES (Δ40, Δ160), or transcription from internal promoter (Δ133, Δ160) [61][62][65,66] (Figure 3). The co-expression of so many isoforms and the difficulty of distinguishing between them using available molecular biology methods make it challenging to evaluate their role in cancer.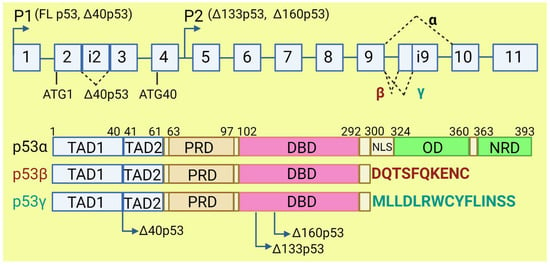
Figure 3. The human TP53 gene and p53 isoforms. (Upper panel): schematic representation of TP53 gene. Exons are numbered from 1 to 11. Alternative splicing of introns i2 and i9 provides alternative p53 isoforms. P1 and P2: alternative promoters. ATG1 and ATG40: alternative transcription start sites. (Lower panel): representation of p53 domains. TAD: transactivation domain; PRD: proline rich domain; DBD: DNA binding domain; NLS: nuclear localization signal; OD: oligomerization domain; NRD: negative regulation domain. Isoforms β and γ lack C-terminal domains, including OD, replaced by DQTSFQKENC and MLLDLRWCYFLINSS, respectively. Isoforms Δ40p53, Δ133p53, and Δ160p53 have a deletion from N-terminus.
