Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Yongqiang Wen and Version 3 by Peter Tang.
Baicalin is one of the most abundant flavonoids found in the dried roots of Scutellaria baicalensis Georgi (SBG) belonging to the genus Scutellaria. Baicalin is demonstrated to have anti-inflammatory, antiviral, antitumor, antibacterial, anticonvulsant, antioxidant, hepatoprotective, and neuroprotective effects.
- baicalin
- bioavailability
- drug interaction
- anti-inflammatory activity
- pharmacokinetics
1. Introduction
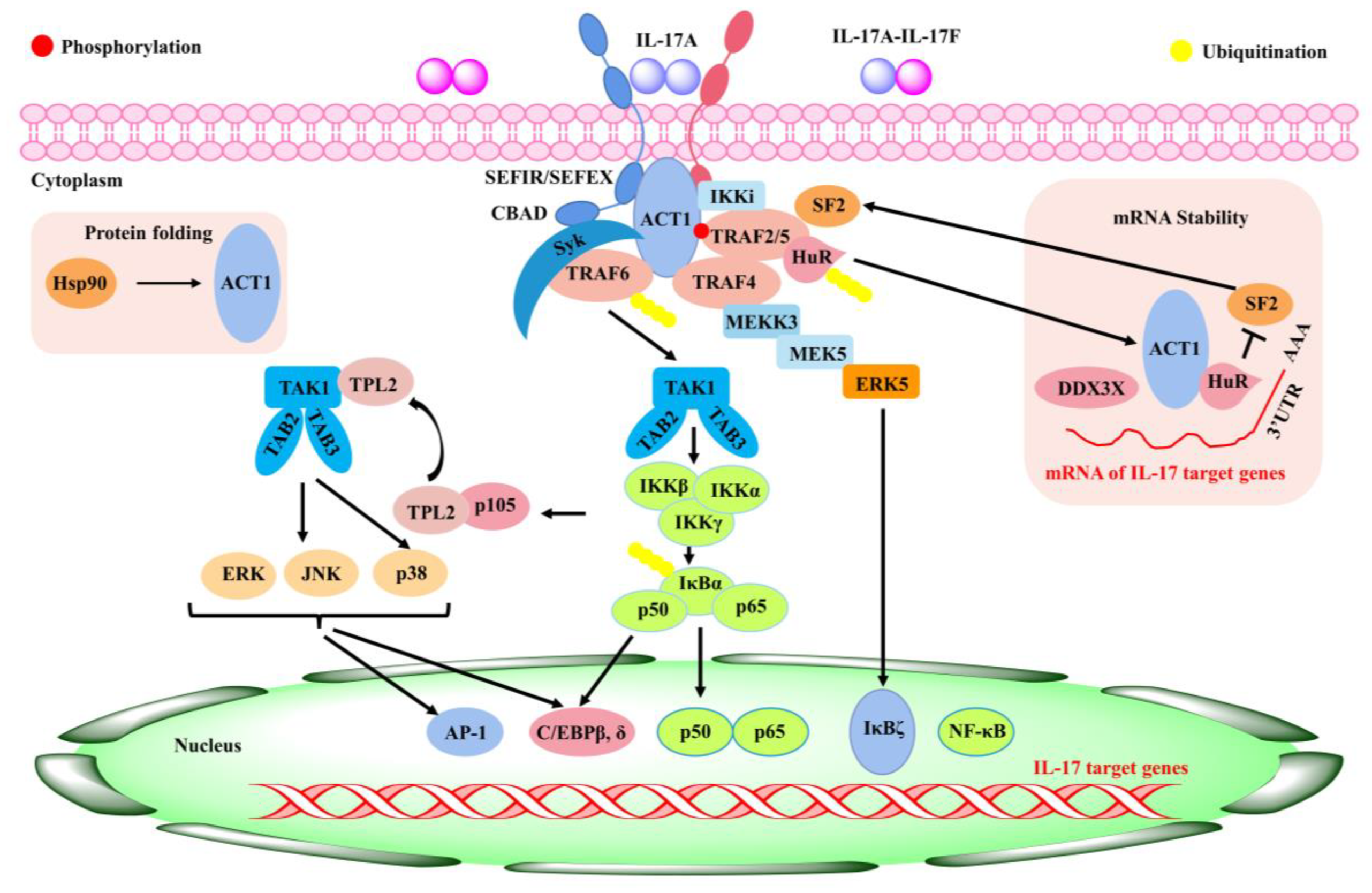
Scutellaria baicalensis Georgi (SBG), belonging to the genus Scutellaria, grows mainly in Asia, including China, Mongolia, Japan, Korea, and Siberia [1]. In China, SBG is regarded as an authentic traditional medicinal herb that grows widely in desert areas and sunny grassy slopes at altitudes of 60–2000 m [2]. It is mainly found in northern provinces of China such as Hebei, Shandong, Shanxi, and Inner Mongolia [3]. Based on SBG’s growing season and cycle characteristics, Xu et al. (2020) determined that the quality of SBG harvested in autumn and cultivated for three years was the best [2]. The primary active constituents of SBG encompass baicalin, baicalein, wogonin, Han baicalin, and Han baicalein [4]. In the field of clinical practice, Scutellaria baicalensis Georgi is frequently employed to treat inflammatory disorders, influenza, diarrhea, jaundice, headache, and abdominal pain [5]. The broad-spectrum pharmacological effect of SBG in diminishing various kinds of diseases is mainly through regulating host immunity [6]. In addition, SBG was reported to have effects such as enhancing immunity, anti-aging, protecting the liver, and anti-osteoporosis [7][8][7,8].
Flavonoids are a class of compounds that are widely distributed in various vegetables and fruits [9]. The consumption of flavonoid-rich fruits and vegetables could reduce the risk of inflammatory diseases [9]. In vivo and in vitro analyses have confirmed that certain flavonoids play a therapeutic effect on diseases. For example, baicalin (7-glucuronic acid, 5,6-dihydroxy-flavone, C21H18O11) has been widely used in recent years for the development of pharmaceutical formulations and the treatment of certain diseases [10]. Baicalin, also known as begalin, is formed by combining the C7 hydroxyl group of baicalein with glucuronic acid [10] (Figure 1). Baicalin is a light-yellow powder under normal conditions; it is bitter in taste, insoluble in alcohols, and soluble in chloroform, nitrobenzene, dimethyl sulfoxide, etc. [11][12][13][11,12,13]. For the function of baicalin, studies have demonstrated that baicalin, containing most of the pharmacological function of SBG in modulating host immunity, plays a therapeutic role in neuroinflammation, enteritis, pneumonia, secondary inflammation, and other diseases in the clinic [14][15][16][14,15,16]. Inflammation is an immune response to invasiveness, which aims to clear away invasive pathogens and initiates tissue repair [17]. Although inflammation has this protective function, inappropriate inflammation can trigger damage to the body and induce the development of diseases [18]. Baicalin has been demonstrated to have anti-inflammatory and immunomodulatory functions, most of which are regulated by inhibiting the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway and nucleotide-binding oligomerization domain-like receptor pyrin domain protein 3 (NLRP3) inflammasome as well as suppressing pro-inflammatory factor expression, such as interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor α (TNF-α), cyclooxygenase 2 (COX-2), inducible nitric oxide synthase (iNOS), etc. [1][17][1,17].
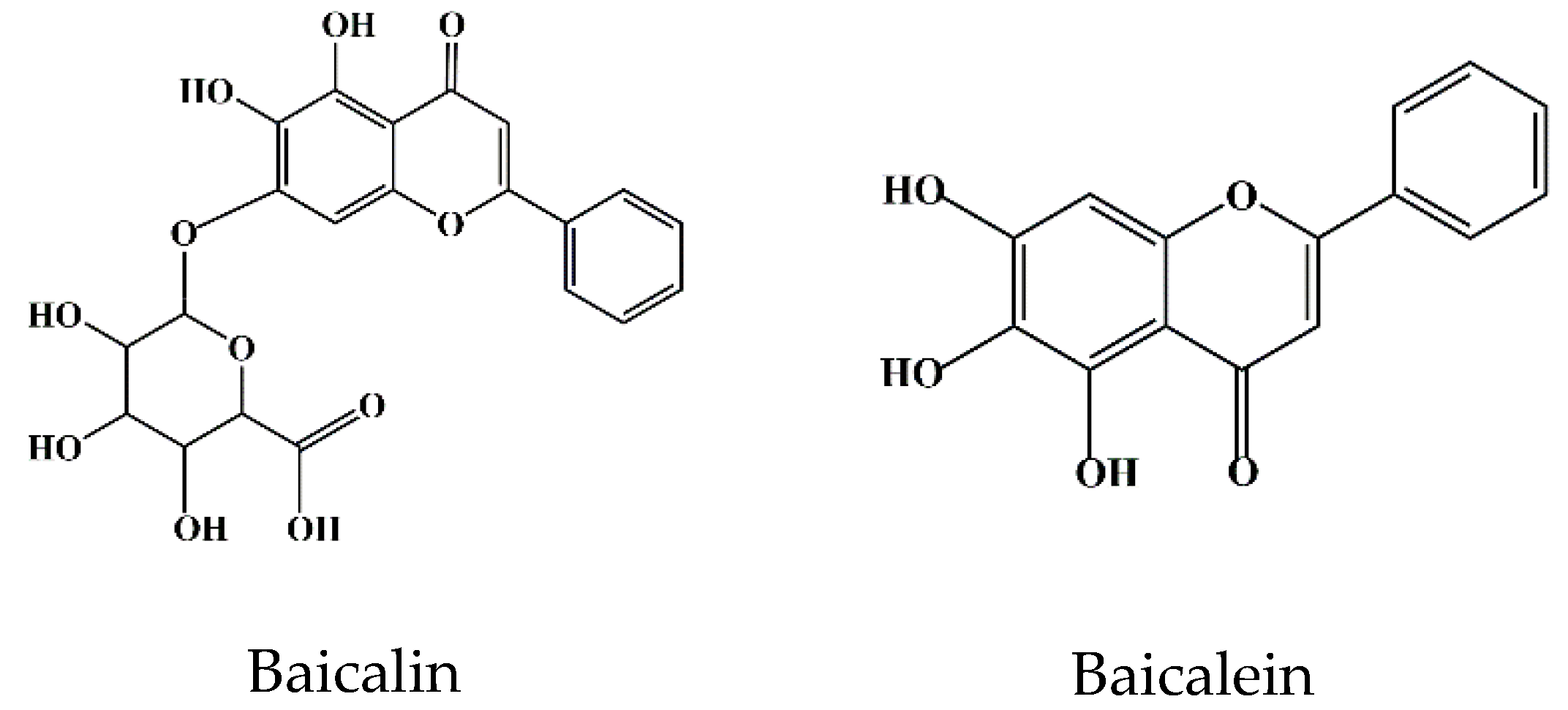
Figure 1. Chemical structure of baicalin and baicalein.
In summary, the pharmacological effects of baicalin are closely related to inhibiting inflammatory reactions. In this entry, the basic physicochemical properties of baicalin and its molecular and immune regulatory mechanisms in the prevention and treatment of inflammatory diseases are reviewed.
In summary, the pharmacological effects of baicalin are closely related to inhibiting inflammatory reactions. In this paper, the basic physicochemical properties of baicalin and its molecular and immune regulatory mechanisms in the prevention and treatment of inflammatory diseases are reviewed.
2. Bioavailability of Baicalin
In pharmacological studies, bioavailability refers to the degree of drug absorbed into the systemic circulation, reflecting the percentage of drug absorbed by the gastrointestinal tract to the oral amount [19]. Generally, the permeability coefficient of drugs with 1% absorption is about 1.0 × 10−6 cm/s. The permeability coefficient of drugs absorbed between 1% and 100% ranges from 1.0 × 10−6 to 0.1 cm/s, and the permeability coefficient of drugs and peptides absorbed less than 1% is below 1.0 × 10−7 cm/s [20].
Its low water solubility (67.03 ± 1.60 μg/mL) and permeability (0.037 × 10−6 cm/m) determine that baicalin cannot be transported by passive diffusion into the host cell lipid bilayer, which results in poor absorption and the low bioavailability of baicalin [21]. Contrarily, baicalein, a glycoside form of baicalin with good permeability and lipophilicity, can be well absorbed by the gastrointestinal tract [22]. Studies have shown that there is an interconversion between baicalin and baicalein during the absorption process of baicalin [22][23][24][22,23,24]. In particular, after drug administration in animals, baicalin could be hydrolyzed to baicalein by β-glucuronidase derived from intestinal bacteria, and baicalein could be recovered to baicalin by uridine 5′-diphosphate (UDP) glucuronide transferase (UGT) circulating in vivo [25]. This mutual conversion maximizes the full pharmacodynamic functions of baicalin [25]. Due to the better absorption of baicalein compared to baicalin, the conversion of baicalin to baicalein is a key step for the absorption of baicalin in the intestine. Furthermore, the intestinal microbiota was reported to be associated with the conversion of baicalin to baicalein [26]. For instance, the intestinal absorption of baicalin in germ-free rats was significantly reduced compared to conventional rats [26], suggesting that the presence of intestinal flora was conducive to the body’s absorption and utilization of baicalin. In other words, only a small proportion of baicalin is absorbed by the physical body as a raw component, and most of it is hydrolyzed into baicalein by bacteria and absorbed by the physical body. Human serum albumin (HSA) is the main transport medium through which flavonoids (for example, baicalin, catechin, quercetin, etc.) are absorbed and utilized by the physical body [27]. The rate of transport and volume distribution of baicalin in the host depends on the binding degree of baicalin to HSA [28]. Especially, it was confirmed that, based on the area under the time–concentration curve, the relative absorption rate of baicalin was approximately 65% [29]. Further pharmacokinetic analysis showed that the peak concentration of baicalin in rat serum was lower than that of baicalein [30]. To improve the bioavailability of baicalin, investigators focused on the development of new formulations of baicalin, such as solid nanocrystal nano-emulsions, solid–liquid nanoparticles, liposome formulations, phospholipid complex hydrogels, etc. [31][32][33][31,32,33]. The application development of these new technologies and products aims to improve the solubility and absorption of baicalin.
3. Toxicity of Baicalin
Clearing the safe dose range and action time of drugs is crucial for drug properties, as these directly threaten animals’ safety. The determination of baicalin’s safe dosage range and its duration of action holds paramount significance across diverse animal and cellular models [34]. To investigate whether baicalin was involved in the regulation of hepatic insulin resistance and gluconeogenic activity, an ex in vivo study showed that intraperitoneal injection of baicalin (50 mg/kg) not only resulted in body weight loss and insulin resistance (Homeostatic model assessment for insulin resistance, HOMA-IR) in obese mice (C57BL/6J) but also reduced glucose intolerance and hyperglycemia. In this study, they also showed that baicalin was not toxic to mice’s liver [35]. In line, baicalin was found to effectively alleviate the development of obesity by inhibiting the expression of p-p38 mitogen-activated protein kinase (MAPK), phosphorylation cyclic adenosine 3′,5′-monophosphate (cAMP) response binding protein (p-CREB), forkhead transcription factor forkhead box O1A (Foxo1), peroxisome proliferator-activated receptor γ coactivators 1α (PGC-1α), phosphoenolpyruvate carboxykinase (PEPCK), and glucose-6-phosphatase (G6Pase) in the liver of obese mice and hepatocytes [35]. Another study performed by Shi et al. (2020) demonstrated that liver toxicity induced by acetaminophen (APAP) in mice was effectively reduced at 6 h, 12 h, and 18 h after baicalin administration, and the necrotic area of liver cells was significantly reduced in mice administrated with 40 mg/kg baicalin [36]. These results indicate that baicalin at a safe dose not only has no cytotoxicity in mice but can also reduce lipotoxicity. In line with this, baicalin was reported to effectively inhibit the occurrence of lipopolysaccharide (LPS)-induced hepatitis in chickens treated with 50, 100, and 200 mg/kg baicalin [37]. An oxidative stress study in non-alcoholic fatty liver disease (NAFLD), revealed that baicalin at concentrations ranging from 0.01 nM to 100 μM did not exhibit any cytotoxic effects on HepG2 cells at 24 h and 48 h, as determined by the cholecystokinin (CCK)-8 assay [38].
Baicalin has a significant inhibitory effect on endoplasmic reticulum (ER) stress. For instance, baicalin (12.5 μM and 25 μM) was found to effectively inhibit the expression of ER stress marker phosphorylated inositol-requiring enzyme 1α (p-IRE1α) and reverse palmitic acid (PA)-induced apoptosis and reactive oxygen species (ROS) production [38]. The main mechanism might be because baicalin reduced PA-induced cytotoxicity by inhibiting ER stress and the activation of thioredoxin-interacting protein (Txnip)/NLRP12 inflammasome [39]. These studies provide a new theoretical basis for the clinical application of baicalin and further improve the cytotoxicity study of baicalin. From a toxicological point of view, baicalin is more toxic than baicalein [39]. Interestingly, intestinal microorganisms might have a protective effect on hepatotoxicity caused by baicalin [39]. To support this, incubation of baicalin with fecal enzymes diminished the cytotoxicity to hepatocellular carcinoma (HepG2) cells in a dose-dependent manner, suggesting that the conversion from baicalin to baicalein by human fecal enzymes protects against baicalin-induced cytotoxicity to HepG2 cells [40][41][40,41]. These studies pave a new theoretical basis for the clinical application of baicalin and further improve the cytotoxicity studies of baicalin.