Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Elena Moretti.
Spermatozoa are highly differentiated cells that produce reactive oxygen species (ROS) due to aerobic metabolism. Below a certain threshold, ROS are important in signal transduction pathways and cellular physiological processes, whereas ROS overproduction damages spermatozoa. Sperm manipulation and preparation protocols during assisted reproductive procedures—for example, cryopreservation—can result in excessive ROS production, exposing these cells to oxidative damage. Human spermatozoa represent an ideal cell model to test numerous compounds in vitro, including antioxidants that can be used as supplements to improve the outcome of these procedures.
- antioxidants
- human sperm in vitro
- oxidative stress
1. Introduction
Infertility is a global health issue. It is defined as the failure to achieve a pregnancy after 12 months or more of regular, unprotected sexual intercourse [1]. It is estimated that 8–12% of couples worldwide are infertile [2] and resort to fertility medical treatment. Both of the partners of a couple can be responsible for non-conception. In at least 50% of cases, a male infertility factor is involved, isolated or in combination with a female factor [3,4][3][4]. There are numerous causes and risk factors contributing to the increasing incidence of male infertility [2]. Many of them share oxidative stress (OS) as a common pathway, including varicocele [5], genitourinary infection and inflammation [6,7][6][7]. In this scenario, assisted fertilisation technologies (ART) represent the treatment of choice for many couples facing infertility problems. The use of these techniques implies gamete handling in the laboratory and the exposure of gametes to atmospheric oxygen. In addition, semen laboratory processing such as centrifugation; cryopreservation; exposure to visible light; and the variation in oxygen tension, pH and temperature [8,9][8][9] enhance the production of reactive oxygen species (ROS).
Spermatozoa are highly differentiated cells that produce ROS as consequence of aerobic metabolism. Below a certain threshold, ROS play a key role in signal transduction pathways and cellular physiological processes. In particular, ROS are necessary for sperm motility, capacitation, the acrosome reaction and oocyte interaction [10,11][10][11]. On the contrary, ROS overproduction and the consequent antioxidant imbalance can damage the cellular structure [12], including the membrane, particularly rich in polyunsaturated fatty acids (PUFA), and molecules such as DNA and proteins [13,14,15,16][13][14][15][16].
Assuming that external laboratory conditions are optimal, a strategy to minimise OS during sperm manipulation can include the treatment of the patients with antioxidants even though the real efficacy of in vivo supplementation is still debated [16,17,18][16][17][18]. Another very interesting strategy includes the supplementation of media with antioxidants. Many in vitro studies have investigated the scavenging ability of antioxidant compounds against OS induced in human sperm [9] and many other studies have successfully used antioxidants to supplement media applied in semen cryopreservation [19,20][19][20].
Human spermatozoa represent a model for an in vitro study because they are motile, and the motility is a parameter that is easy to evaluate; they are nearly transcriptionally and translationally silenced; they do not have DNA-repair activity; and they lack intracellular antioxidant protection. Thus, they depend on and are deeply influenced by the external environment.
2. Structure of Human Spermatozoa
Human spermatozoa are highly differentiated and polarised cells made up of a head and a flagellum joined by a connecting piece, all of which is enveloped by the plasma membrane (Figure 1).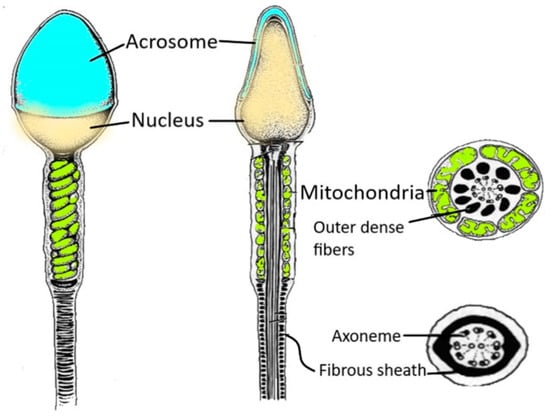
Figure 1. Spermatozoon structure. The figure shows the various regions of a human spermatozoon. Starting from the head region, it is possible to distinguish the acrosome and the nucleus. The sperm flagellum contains the axoneme and periaxonemal structures such as outer dense fibres and a fibrous sheath.
3. Sperm and ROS: An Ambivalent Relationship
Spermatozoa represent a perfect example of the oxygen paradox [48,49][36][37]. Indeed, oxygen homeostasis and the maintenance of a redox steady state are critical for spermatozoa. Spermatozoa generate ROS because they need them for several physiological processes (Figure 2). On the other hand, when ROS production overcomes the antioxidant defences, there are detrimental effects to the sperm membrane, proteins and DNA (Figure 2), which have a negative impact on male fertility [50][38].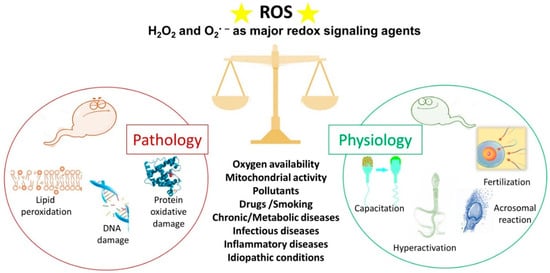
Figure 2. The double face of reactive oxygen species (ROS). Under physiological conditions, ROS regulate the sperm mechanisms involved in capacitation, hyperactivation, the acrosome reaction and fertilisation. On the contrary, some pathophysiological conditions can increase ROS levels over the physiological threshold, generating a condition of oxidative stress that leads to lipid peroxidation, DNA damage and protein oxidative damage. The stars represent ROS.
3.1. ROS and Sperm Physiology
ROS play an important role in sperm physiology because they regulate several intracellular pathways, thus modulating the activation of different transcription factors [50,51,52][38][39][40]. First, ROS are responsible for the stability and compaction of sperm chromatin during epididymal transit and storage. They act as oxidising agents and allow for the formation of disulphide bonds between cysteine residues of protamines, arginine-rich proteins that replace histones during spermiogenesis. Chromatin folding is a crucial event in protecting the paternal genome as the spermatozoa travel through the female reproductive tract [27]. One of the most important roles of ROS in sperm physiology (Figure 2) concerns the process of capacitation by which spermatozoa undergo dramatic changes in the membrane composition and acquire hyperactivated motility, leading to the acrosome reaction and fertilisation [53,54][41][42]. The molecular processes behind capacitation include an increase in pH, Ca2+ and HCO3− influx, efflux of cholesterol from the plasma membrane, an increase in cyclic adenosine monophosphate (cAMP) concentration; and protein hyper-phosphorylation [55,56][43][44]. During capacitation, the concentration of O2•−, H2O2, nitric oxide and peroxynitrite increase progressively. These ROS stimulate the activation of adenylate cyclase that drives the increase in cAMP. This second messenger activates protein kinase A (PKA) that triggers a massive tyrosine phosphorylation cascade, inhibiting tyrosine phosphatase [57,58][45][46]. Redox signalling occurs alongside modification of thiol groups of proteins of the plasma membrane and the inner sperm compartments [53][41]. As a part of the capacitation process, the hyperactivated motility triggered by calcium signalling [59][47] is characterised by a high amplitude and extremely asymmetrical beating pattern of sperm flagellum, as well as lateral head displacement. These motility modifications are dependent on ROS-mediated tyrosine phosphorylation of flagellar protein and are needed to make spermatozoa that can penetrate the cumulus oophorous and zona pellucida and fertilise the oocyte [60,61][48][49]. In addition, the acrosome reaction, the universal requisite for sperm–egg fusion, is influenced by ROS [62][50] that increase membrane fluidity. In fact, spermatozoa exposed to H2O2 show an enhanced acrosome reaction and an increased ability to fuse with oocyte [63][51]. Finally, the involvement of ROS in sperm function and physiology is supported by the observations that antioxidants can alter sperm maturation and, in particular, catalase or superoxide dismutase can inhibit sperm capacitation and the acrosome reaction [64][52].3.2. ROS and Sperm Pathology
In recent decades, OS has emerged as a one of the main causes of altered sperm function [65][53]. Spermatozoa represent an easy target for free radical attack due to the high content of unsaturated fatty acids in their membranes, the limited ability to repair DNA damage and the virtual lack of cytoplasm. Moreover, spermatozoa are poor in antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase, as well as peroxiredoxins [66][54] that protect most cells from oxidative damage. To compensate the scarce presence of cellular antioxidants, the seminal plasma is rich in antioxidant enzymes and radical scavengers, among them glutathione peroxidase, glutathione-S-transferase, catalase and superoxide dismutase [67][55]. In addition, other hydrophilic compounds as uric acid, hypotaurine, tyrosine, polyphenols, vitamin C, ergothioneine and glutathione, and hydrophobic scavengers as trans-retinoic acids, trans-retinols, α-tocopherol, carotenoids and coenzyme Q10 are present [68][56]. The antioxidant properties of seminal plasma are important to balance the presence of ROS that are not just from the physiological production by spermatozoa. Human semen contain various amounts of immature spermatozoa, germinal cells, leucocytes, macrophages and epithelial cells. Among them, the main contributors to OS are leucocytes and immature spermatozoa with large cytoplasmic residues, but in physiological conditions, the redox balance is maintained by antioxidants contained in seminal plasma [69][57]. However, leucocytospermia occurs when the peroxidase-positive leucocyte concentration exceeds 1 × 106/mL [1]. In this pathology, leucocytes produce a hundred times as much ROS as what they would in physiological conditions and the antioxidant power of the seminal plasma is not sufficient to counteract free radicals, leading to OS [70][58]. Immature spermatozoa fail to extrude the cytoplasm during maturation and the residual cytoplasm allows for the production of NADPH from glucose-6-phosphate (G6PDH) via the hexose monophosphate shunt [71,72][59][60]. NADPH generates ROS by using two different pathways: via NADPH oxidase, a membrane bound enzyme that produces the O2•− by oxygen, and via NADPH dehydrogenase, which is responsible for redox reactions in the mitochondria. The enhanced ROS production triggered by immature spermatozoa is responsible for OS propagation to maturing normal spermatozoa during epididymal transit [73][61]. During recent decades, a growing body of evidence has revealed the role of altered redox balance in seminal plasma, sperm alterations and male infertility [12,72,74][12][60][62]. OS is enhanced in situations when non-physiological ROS levels overwhelm the natural scavenger systems. These situations can be represented by primary pathologies affecting the male reproductive system, including varicocele [75][63], bacterial and viral infections, inflammation and leucocytospermia; chronic pathologies such as diabetes and cancer [72,76][60][64]; and environmental and lifestyle factors such as use of drugs, smoking, pollution and radiation (Figure 2). In these conditions, the unconjugated double bonds of PUFA in the sperm membrane are attacked by ROS, producing lipid hydroperoxides and its secondary decomposition product, aldehydes [71][59]. These highly reactive by-products produced by lipid peroxidation react with proteins and DNA and alter the proteins of the electron transport chain to induce mitochondrial dysfunction, enhancing the production of mitochondrial ROS in a self-perpetrating mechanism [9,71,77][9][59][65]. The most evident effect of OS and lipid peroxidation on spermatozoa is the loss of motility by inhibiting energy generation and the decrease in vitality as observed when sperm are frozen and thawed, two processes that boost ROS production [71][59]. The relevance of lipid oxidative damage to sperm conditions has been highlighted by the detection, quantification or immunolocalization of the specific end products of lipid peroxidation (aldehydes and oxygenated metabolites of PUFA) [78,79][66][67]. OS is a major cause of DNA damage in mammalian spermatozoa. Aitken and De Iuliis [80][68] proposed a two-step mechanism for the origin of oxidative DNA damage in spermatozoa. The first phase occurs during spermiogenesis and leads to defective protamination and compaction of sperm chromatin, making DNA more vulnerable to ROS attack. In normal conditions, chromatin compaction is mandatory to protect paternal DNA—this stabilisation makes the spermatozoa resistant to oxidative damage. The second phase is referred to a direct oxidative insult on the DNA due to increased ROS generation by sperm and a loss of extracellular antioxidant protection. The evident ROS effects on sperm nuclear DNA include DNA fragmentation, chromatin cross-linking, base-pair modifications and chromosomal microdeletions [11]. In addition, paternal aging plays a negative effect on sperm parameters and induces a ROS-related DNA fragmentation. These alterations negatively influence the reproductive outcome and offspring health documented for cancer, genetic and congenital diseases, chromosomal alterations and others [81][69].4. Human Spermatozoa as a Model for In Vitro Studies
There are many reasons why human spermatozoa represent a model for in vitro studies (Figure 3). First, the easy collection of spermatozoa from fertile men can guarantee abundant cellular material. Spermatozoa are differentiated cells with features and specific functions that enable them to reach the oocyte and to fertilise it. The first peculiar characteristic of spermatozoa is their motility, which enables them to reach and fertilise the oocyte. This motility is guaranteed by the ability to obtain energy by both mitochondrial oxidative phosphorylation and glycolysis, the enzymes for which are located along the FS [33].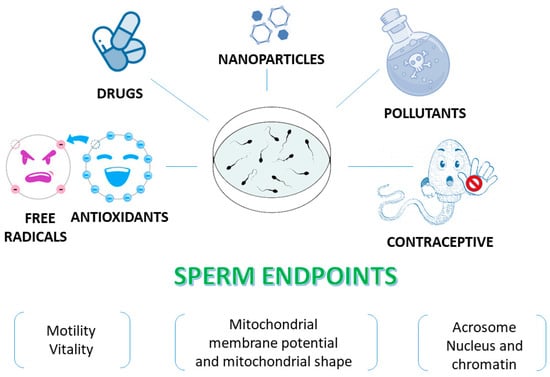
Figure 3. Human sperm as an in vitro model to test various compounds. Sperm endpoints such as motility, vitality, mitochondrial membrane potential, the acrosome status and DNA integrity can be evaluated when human spermatozoa are used as an in vitro model to test effects of toxic compounds, natural extracts and molecules.
References
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021.
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333.
- Nieschlag, E.; Lenzi, A. The conventional management of male infertility. Int. J. Gynaecol. Obstet. 2013, 123 (Suppl. S2), S31–S35.
- Leslie, S.W.; Soon-Sutton, T.L.; Khan, M.A.B. Male infertility. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Wang, K.; Gao, Y.; Wang, C.; Liang, M.; Liao, Y.; Hu, K. Role of oxidative stress in varicocele. Front. Genet. 2022, 13, 850114.
- Pellati, D.; Mylonakis, I.; Bertoloni, G.; Fiore, C.; Andrisani, A.; Ambrosini, G.; Armanini, D. Genital tract infections and infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 140, 3–11.
- Wang, S.; Zhang, K.; Yao, Y.; Li, J.; Deng, S. Bacterial infections affect male fertility: A focus on the oxidative stress-autophagy axis. Front. Cell Dev. Biol. 2021, 9, 727812.
- Walczak-Jedrzejowska, R.; Wolski, J.K.; Slowikowska-Hilczer, J. The role of oxidative stress and antioxidants in male fertility. Cent. Eur. J. Urol. 2013, 66, 60–67.
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Longobardi, S.; Di Rella, F.; Adiga, S.K.; Talevi, R. Sperm oxidative stress during in vitro manipulation and its effects on sperm function and embryo development. Antioxidants 2021, 10, 1025.
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10.
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab J. Urol. 2019, 17, 87–97.
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male infertility and oxidative stress: A focus on the underlying mechanisms. Antioxidants 2022, 11, 306.
- Ford, W.C. Regulation of sperm function by reactive oxygen species. Hum. Reprod. Update 2004, 10, 387–399.
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 2014, 28, 684–703.
- Lone, S.A.; Mohanty, T.K.; Baithalu, R.K.; Yadav, H.P. Sperm protein carbonylation. Andrologia 2019, 51, e13233.
- Agarwal, A.; Maldonado Rosas, I.; Anagnostopoulou, C.; Cannarella, R.; Boitrelle, F.; Munoz, L.V.; Finelli, R.; Durairajanayagam, D.; Henkel, R.; Saleh, R. Oxidative stress and assisted reproduction: A comprehensive review of its pathophysiological role and strategies for optimizing embryo culture environment. Antioxidants 2022, 11, 477.
- Martin-Hidalgo, D.; Bragado, M.J.; Batista, A.R.; Oliveira, P.F.; Alves, M.G. Antioxidants and male fertility: From molecular studies to clinical evidence. Antioxidants 2019, 8, 89.
- Symeonidis, E.N.; Evgeni, E.; Palapelas, V.; Koumasi, D.; Pyrgidis, N.; Sokolakis, I.; Hatzichristodoulou, G.; Tsiampali, C.; Mykoniatis, I.; Zachariou, A.; et al. Redox balance in male infertility: Excellence through moderation—“Μέτρον ἄριστον”. Antioxidants 2021, 10, 1534.
- Amidi, F.; Pazhohan, A.; Shabani Nashtaei, M.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank 2016, 17, 745–756.
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The role of resveratrol in mammalian reproduction. Molecules 2020, 25, 4554.
- Abou-Haila, A.; Tulsiani, D.R. Mammalian sperm acrosome: Formation, contents, and function. Arch. Biochem. Biophys. 2000, 379, 173–182.
- Bowker, Z.; Goldstein, S.; Breitbart, H. Protein acetylation protects sperm from spontaneous acrosome reaction. Theriogenology 2022, 191, 231–238.
- Fujihara, Y.; Murakami, M.; Inoue, N.; Satouh, Y.; Kaseda, K.; Ikawa, M.; Okabe, M. Sperm equatorial segment protein 1, SPESP1, is required for fully fertile sperm in mouse. J. Cell Sci. 2010, 123 Pt 9, 1531–1536.
- Kashir, J.; Heindryckx, B.; Jones, C.; De Sutter, P.; Parrington, J.; Coward, K. Oocyte activation, phospholipase C zeta and human infertility. Hum. Reprod. Update 2010, 16, 690–703.
- Saleh, A.; Kashir, J.; Thanassoulas, A.; Safieh-Garabedian, B.; Lai, F.A.; Nomikos, M. Essential role of sperm-specific PLC-zeta in egg activation and male factor infertility: An update. Front. Cell Dev. Biol. 2020, 8, 28.
- Ward, W.S. Organization of sperm DNA by the nuclear matrix. Am. J. Clin. Exp. Urol. 2018, 6, 87–92.
- Ribas-Maynou, J.; Nguyen, H.; Wu, H.; Ward, W.S. Functional aspects of sperm chromatin organization. Results Probl. Cell Differ. 2022, 70, 295–311.
- Chemes, H.E.; Rawe, V.Y. The making of abnormal spermatozoa: Cellular and molecular mechanisms underlying pathological spermiogenesis. Cell Tissue Res. 2010, 341, 349–357.
- Avidor-Reiss, T.; Carr, A.; Fishman, E.L. The sperm centrioles. Mol. Cell Endocrinol. 2020, 518, 110987.
- Zabeo, D.; Heumann, J.M.; Schwartz, C.L.; Suzuki-Shinjo, A.; Morgan, G.; Widlund, P.O.; Höög, J.L. A lumenal interrupted helix in human sperm tail microtubules. Sci. Rep. 2018, 8, 2727.
- Boguenet, M.; Bouet, P.E.; Spiers, A.; Reynier, P.; May-Panloup, P. Mitochondria: Their role in spermatozoa and in male infertility. Hum. Reprod. Update 2021, 27, 697–719.
- Toure, A.; Rode, B.; Hunnicutt, G.R.; Escalier, D.; Gacon, G. Septins at the annulus of mammalian sperm. Biol. Chem. 2011, 392, 799–803.
- Kim, Y.H.; Haidl, G.; Schaefer, M.; Egner, U.; Mandal, A.; Herr, J.C. Compartmentalization of a unique ADP/ATP carrier protein SFEC (Sperm Flagellar Energy Carrier, AAC4) with glycolytic enzymes in the fibrous sheath of the human sperm flagellar principal piece. Dev. Biol. 2007, 302, 463–476.
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377.
- Barrachina, F.; Battistone, M.A.; Castillo, J.; Mallofré, C.; Jodar, M.; Breton, S.; Oliva, R. Sperm acquire epididymis-derived proteins through epididymosomes. Hum. Reprod. 2022, 37, 651–668.
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219.
- Davies, K.J. Oxidative stress: The paradox of aerobic life. Biochem. Soc. Symp. 1995, 61, 1–31.
- Mannucci, A.; Argento, F.R.; Fini, E.; Coccia, M.E.; Taddei, N.; Becatti, M.; Fiorillo, C. The impact of oxidative stress in male infertility. Front. Mol. Biosci. 2022, 8, 799294.
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299.
- Takei, G.L.; Tourzani, D.A.; Paudel, B.; Visconti, P.E. Activation of cAMP-dependent phosphorylation pathways is independent of ROS production during mouse sperm capacitation. Mol. Reprod. Dev. 2021, 88, 544–557.
- O’Flaherty, C. Redox regulation of mammalian sperm capacitation. Asian J. Androl. 2015, 17, 583–590.
- De Jonge, C. Biological basis for human capacitation-revisited. Hum. Reprod. Update 2017, 23, 289–299.
- Carrasquel Martínez, G.; Aldana, A.; Carneiro, J.; Treviño, C.L.; Darszon, A. Acrosomal alkalinization occurs during human sperm capacitation. Mol. Hum. Reprod. 2022, 28, gaac005.
- Delgado-Bermúdez, A.; Yeste, M.; Bonet, S.; Pinart, E. A review on the role of bicarbonate and proton transporters during sperm capacitation in mammals. Int. J. Mol. Sci. 2022, 23, 6333.
- Herrero, M.B.; Chatterjee, S.; Lefièvre, L.; de Lamirande, E.; Gagnon, C. Nitric oxide interacts with the cAMP pathway to modulate capacitation of human spermatozoa. Free Radic. Biol. Med. 2000, 29, 522–536.
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Positive role of reactive oxygen species in mammalian sperm capacitation: Triggering and modulation of phosphorylation events. Free Radic. Biol. Med. 2006, 41, 528–540.
- Cordero-Martínez, J.; Jimenez-Gutierrez, G.E.; Aguirre-Alvarado, C.; Alacántara-Farfán, V.; Chamorro-Cevallos, G.; Roa-Espitia, A.L.; Hernández-González, E.O.; Rodríguez-Páez, L. Participation of signaling proteins in sperm hyperactivation. Syst. Biol. Reprod. Med. 2022, 68, 315–330.
- de Lamirande, E.; Gagnon, C. Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radic. Biol. Med. 1993, 14, 157–166.
- Suarez, S.S. Control of hyperactivation in sperm. Hum. Reprod. Update 2008, 14, 647–657.
- de Lamirande, E.; Tsai, C.; Harakat, A.; Gagnon, C. Involvement of reactive oxygen species in human sperm arcosome reaction induced by A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J. Androl. 1998, 19, 585–594.
- Rivlin, J.; Mendel, J.; Rubinstein, S.; Etkovitz, N.; Breitbart, H. Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol. Reprod. 2004, 70, 518–522.
- Wagner, H.; Cheng, J.W.; Ko, E.Y. Role of reactive oxygen species in male infertility: An updated review of literature. Arab J. Urol. 2017, 16, 35–43.
- Aitken, R.J.; Drevet, J.R. The importance of oxidative stress in determining the functionality of mammalian spermatozoa: A two-edged sword. Antioxidants 2020, 9, 111.
- O’Flaherty, C. Peroxiredoxin 6: The protector of male fertility. Antioxidants 2018, 7, 173.
- Micheli, L.; Cerretani, D.; Collodel, G.; Menchiari, A.; Moltoni, L.; Fiaschi, A.I.; Moretti, E. Evaluation of enzymatic and non-enzymatic antioxidants in seminal plasma of men with genitourinary infections, varicocele and idiopathic infertility. Andrology 2016, 4, 456–464.
- Lazzarino, G.; Listorti, I.; Bilotta, G.; Capozzolo, T.; Amorini, A.M.; Longo, S.; Caruso, G.; Lazzarino, G.; Tavazzi, B.; Bilotta, P. Water- and fat-soluble antioxidants in human seminal plasma and serum of fertile males. Antioxidants 2019, 8, 96.
- Aitken, R.J.; Baker, M.A. Oxidative stress, spermatozoa and leukocytic infiltration: Relationships forged by the opposing forces of microbial invasion and the search for perfection. J. Reprod. Immunol. 2013, 100, 11–19.
- Henkel, R.R. Leukocytes and oxidative stress: Dilemma for sperm function and male fertility. Asian J. Androl. 2011, 13, 43–52.
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052.
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018, 50, e13126.
- Gil-Guzman, E.; Ollero, M.; Lopez, M.C.; Sharma, R.K.; Alvarez, J.G.; Thomas, A.J., Jr.; Agarwal, A. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum. Reprod. 2001, 16, 1922–1930.
- Villaverde, A.I.S.B.; Netherton, J.; Baker, M.A. From past to present: The link between reactive oxygen species in sperm and male infertility. Antioxidants 2019, 8, 616.
- Wood, G.J.A.; Cardoso, J.P.G.; Paluello, D.V.; Nunes, T.F.; Cocuzza, M. Varicocele-associated infertility and the role of oxidative stress on sperm DNA fragmentation. Front. Reprod. Health 2021, 3, 695992.
- Signorini, C.; Moretti, E.; Noto, D.; Micheli, L.; Ponchia, R.; Collodel, G. Fatty acid oxidation and pro-resolving lipid mediators are related to male infertility. Antioxidants 2022, 11, 107.
- Aitken, R.J.; Whiting, S.; De Iuliis, G.N.; McClymont, S.; Mitchell, L.A.; Baker, M.A. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J. Biol. Chem. 2012, 287, 33048–33060.
- Moretti, E.; Cerretani, D.; Noto, D.; Signorini, C.; Iacoponi, F.; Collodel, G. Relationship between semen IL-6, IL-33 and malondialdehyde generation in human seminal plasma and spermatozoa. Reprod. Sci. 2021, 28, 2136–2143.
- Moretti, E.; Signorini, C.; Ferretti, F.; Noto, D.; Collodel, G. A study to validate the relevance of semen F2-isoprostanes on human male infertility. Int. J. Environ. Res. Public Health 2022, 19, 1642.
- Aitken, R.J.; De Iuliis, G.N. On the possible origins of DNA damage in human spermatozoa. Mol. Hum. Reprod. 2010, 16, 3–13.
- Jimbo, M.; Kunisaki, J.; Ghaed, M.; Yu, V.; Flores, H.A.; Hotaling, J.M. Fertility in the aging male: A systematic review. Fertil. Steril. 2022, 118, 1022–1034.
- Santiago, J.; Silva, J.V.; Howl, J.; Santos, M.A.S.; Fardilha, M. All you need to know about sperm RNAs. Hum. Reprod. Update 2021, 28, 67–91.
- Vollmer, T.; Ljungberg, B.; Jankowski, V.; Jankowski, J.; Glorieux, G.; Stegmayr, B.G. An in-vitro assay using human spermatozoa to detect toxicity of biologically active substances. Sci. Rep. 2019, 9, 14525.
- Setti, A.S.; Braga, D.P.A.F.; Provenza, R.R.; Iaconelli, A., Jr.; Borges, E., Jr. Oocyte ability to repair sperm DNA fragmentation: The impact of maternal age on intracytoplasmic sperm injection outcomes. Fertil. Steril. 2021, 116, 123–129.
- Collodel, G.; Federico, M.G.; Geminiani, M.; Martini, S.; Bonechi, C.; Rossi, C.; Figura, N.; Moretti, E. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod. Toxicol. 2011, 31, 239–246.
- Eskandari, F.; Momeni, H.R. Silymarin protects plasma membrane and acrosome integrity in sperm treated with sodium arsenite. Int. J. Reprod. Biomed. 2016, 14, 47–52.
- Zhang, L.; Diao, R.Y.; Duan, Y.G.; Yi, T.H.; Cai, Z.M. In vitro antioxidant effect of curcumin on human sperm quality in leucocytospermia. Andrologia 2017, 49, e12760.
- Noto, D.; Collodel, G.; Cerretani, D.; Signorini, C.; Gambera, L.; Menchiari, A.; Moretti, E. Protective effect of chlorogenic acid on human sperm: In vitro studies and frozen-thawed protocol. Antioxidants 2021, 10, 744.
- Amirjannaty, S.; Gashti, N.G.; Mojtahedi, A.; Ashouri, A.; Bahadori, M.H. An in vitro study on the protective effect of melatonin on human sperm parameters treated by cadmium. J. Hum. Reprod. Sci. 2022, 15, 21–26.
- Mottola, F.; Iovine, C.; Carannante, M.; Santonastaso, M.; Rocco, L. In vitro combination of ascorbic and ellagic acids in sperm oxidative damage inhibition. Int. J. Mol. Sci. 2022, 23, 14751.
- Munir, N.; Mahmood, Z.; Shahid, M.; Afzal, M.N.; Jahangir, M.; Ali Shah, S.M.; Tahir, I.M.; Riaz, M.; Hussain, S.; Akram, M.; et al. Withania somnifera chemical constituents’ in vitro antioxidant potential and their response on spermatozoa parameters. Dose Response 2022, 20, 15593258221074936.
- Paul, S.; Kang, S.C. In vitro determination of the contraceptive spermicidal activity of essential oil of Trachyspermum ammi (L.) Sprague ex Turrill fruits. New Biotechnol. 2011, 28, 684–690.
- Fan, Y.; Chen, N.; Wang, M.; Rao, M.; Su, P. In vitro study evaluating the instantaneous treatment of ozonised olive oil on human sperm. Eur. J. Contracept. Reprod. Health Care 2018, 23, 147–153.
- Sha, W.; Chang, X.; Han, Y.; Miao, H.; Yang, Y. In vitro effect of ulipristal acetate on human sperm parameters and function. Pak. J. Pharm. Sci. 2019, 32, 1419–1422.
- Mondal, P.; Maity, R.; Mallick, C. In vitro spermicidal effect of Thevetia Peruviana leaves on human spermatozoa. Andrologia 2022, 54, e14323.
- Marchiani, S.; Tamburrino, L.; Farnetani, G.; Muratori, M.; Vignozzi, L.; Baldi, E. Acute effects on human sperm exposed in vitro to cadmium chloride and diisobutyl phthalate. Reproduction 2019, 158, 281–290.
- Xie, F.; Chen, X.; Weng, S.; Xia, T.; Sun, X.; Luo, T.; Li, P. Effects of two environmental endocrine disruptors di-n-butyl phthalate (DBP) and mono-n-butyl phthalate (MBP) on human sperm functions in vitro. Reprod. Toxicol. 2019, 83, 1–7.
- Chen, C.; Li, B.; Huang, R.; Dong, S.; Zhou, Y.; Song, J.; Zeng, X.; Zhang, X. Involvement of Ca2+ and ROS signals in nickel-impaired human sperm function. Ecotoxicol. Environ. Saf. 2022, 231, 113181.
- Moretti, E.; Terzuoli, G.; Renieri, T.; Iacoponi, F.; Castellini, C.; Giordano, C.; Collodel, G. In vitro effect of gold and silver nanoparticles on human spermatozoa. Andrologia 2013, 45, 392–396.
- Préaubert, L.; Tassistro, V.; Auffan, M.; Sari-Minodier, I.; Rose, J.; Courbiere, B.; Perrin, J. Very low concentration of cerium dioxide nanoparticles induce DNA damage, but no loss of vitality, in human spermatozoa. Toxicol. Vitr. 2018, 50, 236–241.
- Santonastaso, M.; Mottola, F.; Iovine, C.; Cesaroni, F.; Colacurci, N.; Rocco, L. In vitro effects of titanium dioxide nanoparticles (TiO2NPs) on cadmium chloride (CdCl2) genotoxicity in human sperm cells. Nanomaterials 2020, 10, 1118.
- Tan, Z.; Zhou, J.; Chen, H.; Zou, Q.; Weng, S.; Luo, T.; Tang, Y. Toxic effects of 2,4-dichlorophenoxyacetic acid on human sperm function in vitro. J. Toxicol. Sci. 2016, 41, 543–549.
- Xu, B.; Wang, Z.P.; Wang, Y.J.; Lu, P.H.; Wang, L.J.; Wang, X.H. The toxic effect of opioid analgesics on human sperm motility in vitro. Drug Chem. Toxicol. 2013, 36, 205–208.
- Pascarelli, N.A.; Fioravanti, A.; Moretti, E.; Guidelli, G.M.; Mazzi, L.; Collodel, G. The effects in vitro of TNF-α and its antagonist ‘etanercept’ on ejaculated human sperm. Reprod. Fertil. Dev. 2017, 29, 1169–1177.
- Ali Banihani, S.; Al-Khawalde, A.A. Omeprazole does not alter human sperm motility, viability or DNA integrity in vitro. Andrologia 2019, 51, e13260.
- Ito, C.; Toshimori, K. Acrosome markers of human sperm. Anat. Sci. Int. 2016, 91, 128–142.
- Zoppino, F.C.; Halón, N.D.; Bustos, M.A.; Pavarotti, M.A.; Mayorga, L.S. Recording and sorting live human sperm undergoing acrosome reaction. Fertil. Steril. 2012, 97, 1309–1315.
- Farkouh, A.; Salvio, G.; Kuroda, S.; Saleh, R.; Vogiatzi, P.; Agarwal, A. Sperm DNA integrity and male infertility: A narrative review and guide for the reproductive physicians. Transl. Androl. Urol. 2022, 11, 1023–1044.
- Carrageta, D.F.; Freire-Brito, L.; Oliveira, P.F.; Alves, M.G. Evaluation of human spermatozoa mitochondrial membrane potential using the JC-1 dye. Curr. Protoc. 2022, 2, e531.
- van der Horst, G.; Maree, L.; du Plessis, S.S. Current perspectives of CASA applications in diverse mammalian spermatozoa. Reprod. Fertil. Dev. 2018, 30, 875–888.
- Agarwal, A.; Sharma, R.K.; Gupta, S.; Boitrelle, F.; Finelli, R.; Parekh, N.; Durairajanayagam, D.; Saleh, R.; Arafa, M.; Cho, C.L.; et al. Sperm vitality and necrozoospermia: Diagnosis, management, and results of a global survey of clinical practice. World J. Mens Health 2022, 40, 228–242.
More
