Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Carlo Airola and Version 2 by Rita Xu.
The human gut is inhabited by a multitude of bacteria, yeasts, and viruses. A dynamic balance among these microorganisms is associated with the well-being of the human being, and a large body of evidence supports a role of dysbiosis in the pathogenesis of several diseases. Given the importance of the gut microbiota in the preservation of human health, probiotics, prebiotics, synbiotics, and postbiotics have been classically used as strategies to modulate the gut microbiota and achieve beneficial effects for the host. Besides, several molecules not typically included in these categories have demonstrated a role in restoring the equilibrium among the components of the gut microbiota.
- eubiotics
- microbiota
- dysbiosis
- rifaximin
- microbiota modulation
- gastrointestinal diseases
1. Introduction
Interest in the human gut microbiota and its potential impact on human health has significantly increased since the development of metagenomic technologies that allow for the deep sequencing of microbial genomes. Trillions of bacteria live in the gut microbiota of the human digestive system and are established as a very complex environment. Beginning at birth, a wide range of genetic, nutritional, and environmental variables influence the composition of the gut microbiome [1]. After the beginning of the 20th century, Metchnikoff proposed that the human gut microbiome plays a part in both health and sickness [2]. Subsequently, the introduction of new molecular technologies that emerged in metagenomics spread a new perspective on microbiota research. The so-called “microbiota revolution” enabled the discovery of crucial links between pathogenic diseases and gut microbes [3].
The gut microbiota is composed of bacteria (generally including Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia phila), yeasts, and viruses [4][5][6][4,5,6]. The gut bacteria perform a variety of functions, including vitamin production, pathogen defense, immune response stimulation, metabolism regulation and drug absorption [6]. High taxonomic diversity, microbial gene richness, and a stable core microbiota are frequently observed in healthy microbiota communities [6]. However, the relative distribution of microorganisms varies between individuals, even within the same person. Nearly every element of the host can be impacted by the microbiota, and dysbiosis is linked to a wide range of illnesses. In particular, dysbiosis has been implicated in cardiovascular and respiratory diseases, inflammatory bowel diseases, liver disorders, a variety of neoplasms, and metabolic illnesses via the intensification of a chronic inflammatory state [7][8][9][7,8,9]. It has also been suggested that gut microbes play a part in preserving the homeostasis of the gut–brain axis [9]. It has been supposed that different molecular patterns enhance microbiota-associated pathogenic states. Recently, it was discovered that the microbiota produces small molecules called genotoxins. In particular, the family of indolimines generated by the M. morganii strains associated with IBD–colorectal cancer can enhance colon carcinogenesis in mice and increase intestinal permeability [10]. Other small-molecule metabolites of Gram-positive and Gram-negative bacteria, such as those from Clostridium perfringens and Clostridium ramosum strains, directly damage DNA and cause the expression of double-strand break markers—as well as cell-cycle arrest—in epithelial cells [10]. Alterations in intestinal microbiota can therefore be correlated to pathological states, both through a non-specific mechanism of chronic inflammation and through specific molecular patterns (Figure 1); therefore, it is essential to achieve the ability to both modulate complex microbial communities and target individual members within these communities.
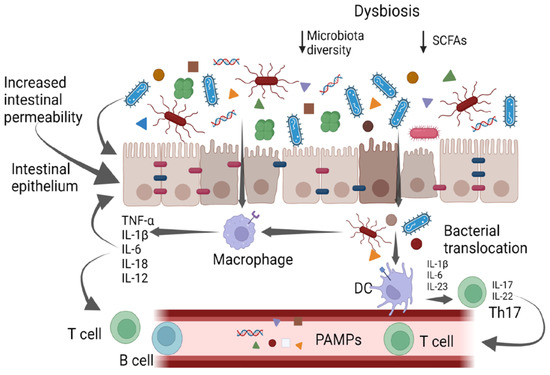
Figure 1. Gut bacteria and systemic inflammation. PAMPs—pathogen-associated molecular patterns; DCs—dendritic cells.
The intestinal mucosa provides a selective, permeable barrier for nutrient absorption and protection from external factors. Pathogens, xenobiotics, and food can disrupt the intestinal barrier, while genetic and immune factors predispose individuals to gut barrier dysfunction. The gut microbiota participates in regulating the integrity and function of the intestinal barrier in a homeostatic balance in various ways: opposing colonization by pathogens, promoting the differentiation of regulatory T (Treg) cells (which induce tolerance to lumen antigens), and stimulating B cells to secrete immunoglobulin (Ig)A to avoid bacterial translocation. Commensals also convert dietary fiber into short-chain fatty acids (SCFAs), which protect the gut barrier in various ways, including by providing energy for colonocytes and stimulating the production of mucus, antimicrobial proteins, and Treg cells. Dysbiosis and chronic disruption of the gut barrier can lead to the translocation of microbial components, the activation of pro-inflammatory patterns, and the production of systemic, low-grade inflammation [11][12][13][11,12,13].
To date, a multitude of molecules with a modulatory effect on gut microbiota have been developed [14][15][16][17][18][14,15,16,17,18]. Most of these agents are prebiotics, probiotics, synbiotics, or postbiotics [14][15][16][17][18][14,15,16,17,18]. However, some molecules known for their antimicrobial effect have been shown to improve the balance of the gut microbiota [19]. Due to their properties, these agents could not be included in the previously mentioned therapeutic groups [19]. Nonetheless, rather than being antimicrobial, their activity may be classified as eubiotic [19].
2. Eubiotics: Drugs to Modulate the Gut Microbiota
Prebiotics and probiotics have historically played a crucial role in the regulation of the gut microbiota. Prebiotics are typically non-digestible food components that selectively encourage the growth and activity of a small number of bacteria in the digestive tract [14]. Probiotics are live microorganisms which, when administered in adequate amounts, confer a health benefit to the host [15]. Synbiotics (a combination of prebiotics and probiotics) and postbiotics (inanimate microorganisms and/or their components that confer a health benefit to the host) have been recently introduced, showing a beneficial effect upon the dysbiotic state [16][17][18][16,17,18]. Nevertheless, pharmacological agents not included in the above-mentioned groups demonstrate an interesting ability to alter microbiota, preventing dysbiosis, with a significant effect on associated diseases. Antimicrobials, natural compounds, and even metabolites of the same gut microbiota have been considered. A new group, eubiotics, has been proposed [20][21][54,55]. All these molecules have a somewhat antimicrobial activity; however, rather than determining a global depletion in bacteria abundance, the antimicrobial activity leads to a modulation of the composition of microbiota, which can be beneficial for the host [20][21][54,55].2.1. Rifaximin: New Perspectives for an Old Antibiotic
During the last half of the 20th century, a number of new antibacterial agents came into clinical use, providing clinicians with a variety of options when treating many types of infectious diseases; among these options, antibiotics played a major role [22][56]. In addition to their beneficial effect against pathogenic bacteria, antibiotics are associated with significant short- and long-term alterations in the composition of the human microbiota [23][57]. The way an antibiotic affects the gut microbiota depends largely on its class, as well as the composition of the microbiota in the gut before the antibiotic is administered [24][58]. The use of an antibiotic results in a decrease in alpha (α) diversity, which measures the variety of bacterial taxa present in a given individual’s microbiota, and in beta (β)-diversity, which is a marker of the homogeneity of bacterial relative abundance [24][25][58,59]. Since suppressing susceptible microbes can create an ecological niche for opportunistic pathogenic bacteria that are resistant to the antibiotic, increasing the host’s susceptibility to post-antibiotic infection, the majority of studies focus on the dysbiotic effect of antibiotics [23][26][27][57,60,61]. Additionally, it has been demonstrated that antibiotics enrich phage-encoded genes, which transfer resistance to the administered drug and unrelated antibiotics and encourage interactions between phages and bacteria, thereby enhancing the exchange of resistance genes [28][62]. Nonetheless, some antibiotics have shown an intriguing function in the clinical modulation of the gut microbiota. Taking into account changes in bacterial abundance, the non-systemic antibiotic rifaximin, which has bactericidal and bacteriostatic activity against both aerobic and anaerobic bacterial species, has demonstrated promising results [29][63]. Rifaximin also has bile-acid-dependent solubility, which increases its effectiveness in the small intestine while inhibiting colonic bacteria only moderately [30][64]. Rifaximin irreversibly binds the bacterial DNA-dependent RNA polymerase, inhibiting bacterial protein synthesis [31][65]. To date, rifaximin is usually administered with beneficial effects in the management of diseases associated with an alteration in the gut microbiota, such as irritable bowel syndrome (IBS), diverticular disease, and hepatic encephalopathy (HE) [32][33][34][66,67,68]. Orally administered rifaximin determines minimal changes in the composition of gut microbiota, promoting the growth of bacterial species with a beneficial impact [29][63]. In addition, rifaximin modulates the inflammatory response by upregulating the expression of NF-kB via the pregnane X receptor [35][36][69,70] and downregulating the pro-inflammatory cytokines interleukin-1B and tumor necrosis factor alpha (TNFα) [37][38][71,72]. As observed in a clinical trial based on a multi-tagged pyrosequencing analysis, rifaximin modulates the networks among several bacteria (Enterobacteriaceae, Bacteroidaceae, Veillonellaceae, Porphyromonadaceae, and Rikenellaceae) and bacterial metabolites when administered to patients with mild HE [39][73]. An analysis of the serum of treated patients revealed an increase in saturated and unsaturated fatty acids [39][73]. Furthermore, rifaximin increased bacterial diversity, the Bacteroidetes/Firmicutes ratio, and the abundance of Faecalibacterium prausnitzii, a butyrate producer with potent anti-inflammatory properties, in a second smaller study involving 15 patients with IBS [40][74]. An analysis of gut microbiota diversity showed that the clinical response was associated with a slight increase in α-diversity [40][74]. Treatment with 1200 mg of rifaximin daily for 10 days increased the abundance of Lactobacilli in another report on 19 patients with various gastrointestinal and liver disorders (inflammatory bowel disorder, IBS, diverticular disease, and HE), with no effects upon the α-diversity of the gut microbiota [20][54]. A recent clinical trial on patients with HE demonstrated that daily treatment with 1200 mg of rifaximin increased the abundance of Proteobacteria while decreasing the abundance of Firmicutes [41][75]. A metagenomic analysis highlighted that a decrease in the prevalence of Veillonella, Haemophilus, Streptococcus, Parabacteroides, Megamonas, Roseburia, Alistipes, Ruminococcus, and Lactobacillus was also associated with rifaximin administration. Despite this trend of bacterial abundance modification, the stability of the gut microbiota was not affected by rifaximin administration, and its minimal antibacterial effect in the intestine was confirmed in this report [41][75]. While the overall composition of the gut microbiota is unaffected by the treatment, rifaximin causes a relative rather than an absolute change in the abundance of these helpful bacteria. This might be because some microbes that are sensitive to rifaximin experienced a minimal reduction in abundance that is not statistically significant while others, such as Lactobacilli, developed resistance [20][54]. Interestingly, Brigidi et al. showed a significant initial decrease in the fecal abundance of Lactobacilli in individuals with mild or severe ulcerative colitis who were receiving a higher dosage of rifaximin (i.e., 1800 mg daily). However, the abundance of Lactobacilli returned after three cycles of therapy that each lasted 10 days. Moreover, Lactobacilli, which were particularly susceptible to rifaximin at the beginning of the study, developed resistance to the drug (to a mean value of 12 μg/mL) [42][76]. In order to improve the pharmacological approach and incorporate it into therapeutic options, it is necessary to investigate the mechanisms that underlie the clinical impact exerted by this beneficial gut microbiota perturbation.2.2. The Multi-Layered Mechanisms of Rifaximin
Rifaximin, as previously mentioned, differs from other antibiotics as it does not affect the gut microbiota’s overall composition. Indeed, rifaximin increases the abundance of beneficial gut bacteria and promotes metabolic modifications, balancing the relationship between the host and the bacteria with a well-recognized positive clinical effect. Although the underlying biological mechanisms are not fully understood, some metabolic networks may be suggested. Rifaximin treatment protected mice exposed to malathion from stress-induced oxidative damage in a pre-clinical study [43][77]. In the gut microbiota of rifaximin-treated mice, acetate- and propionate-producing bacteria, such as Lactobacillus spp., Bacteroides spp., Prevotella spp., Streptococcus spp., Phascolarctobacterium succinatutens, and Negativicutes spp., were found in high concentrations [43][77]. Therefore, treatment with rifaximin effectively increased the production of short-chain fatty acids (SCFAs) by modulating the gut microbiota [44][78]. Dupraz et al. showed that intestinal gamma-delta (γδ) T cells in mice and humans produce less IL-17 and IL-22 when microbiota-produced SCFAs, particularly propionate, are present. This results in a decrease in the inflammatory state [45][79]. Furthermore, the administration of rifaximin was linked to an increase in the expression of peroxisome proliferator-activated receptor gamma coactivator-1 alpha, a key metabolic regulator whose expression is elicited by SCFAs [43][46][77,80]. As a result, SCFAs might be the means through which rifaximin reduces systemic inflammation and exerts its beneficial effect. Another preclinical model highlighted rifaximin’s function in controlling systemic inflammatory responses. By inhibiting the activation of the TLR-4/NF-B signaling pathway and downregulating inflammatory factors such as TNF-α, IL-6, IL-17A, and IL-23, rifaximin significantly diminished the severity of clinical disease in mice with proteoglycan-induced ankylosing spondylitis. In this instance, a microbiological analysis showed an increase in Lactobacillus and Bacteroides, with a higher Bacteroidetes/Firmicutes ratio [47][81]. Additionally, in vitro studies performed in human gut epithelial cells indicated that rifaximin decreases apoptosis and increases tight junction protein expression by activating the TLR4, MyD88, and NF-kB pathways [48][82]. Meanwhile, increased populations of bacteria such as Lactobacilli have been shown to reduce the production of pro-inflammatory cytokines and TNF-α to prevent the spread of pathogenic bacteria and to modulate intestinal permeability [49][50][51][83,84,85]. As a result, rifaximin not only creates an anti-inflammatory environment by regulating intestinal metabolic pathways but also helps to reestablish the function of the intestinal barrier by reducing intestinal permeability, preventing the translocation and overgrowth of bacteria that could be harmful to the individual [21][55]. Patel et al. proposed that these preclinical implications could be transferred to a human model in a recent randomized controlled clinical trial [52][86]. In comparison to the placebo group, 90 days of rifaximin treatment decreased the plasma levels of tumor necrosis factor alpha (TNF-α) and circulating neutrophil TLR-4 expression in patients with cirrhosis and hepatic encephalopathy [52][86]. Additionally, metagenomic quantification techniques showed that rifaximin reduced the levels of sialidase-rich species that degrade mucin (i.e., Streptococcus spp., Veillonella atypica and parvula, Akkermansia, and Hungatella), preventing the so-called “oralization” of the gut microbiota [52][86]. These bacteria are present in the oral microbiota, but they are also more prevalent in the gut microbiota in conditions such as cirrhosis [53][87]. Sialidase alters the intestinal permeability by degrading O-glycans in the gut mucin barrier. Rifaximin also promoted an intestinal microenvironment rich in TNF-α and interleukin-17E (IL-17) [52][86]. It has been demonstrated that IL-17E (also known as IL-25), unlike other isoforms of IL-17, is implicated in the integrity of the gut barrier [54][55][88,89]. Intestinal tuft cells produce IL-17E in response to indole-acetic propionic acid, a tryptophan metabolite involved in gut homeostasis and anti-inflammatory pathways mediated by IL-10 [54][55][88,89]. It is also intriguing to note that plasma TNF-α levels decreased while intestinal TNF-α increased, demonstrating how the maintenance of gut homeostasis and the control of systemic inflammation can be achieved by increasing an inflammatory cytokine in a specific microenvironment. Clinically, rifaximin treatment resulted in a recovery of neurocognitive function, in addition to the complete resolution of hepatic encephalopathy. Additionally, rifaximin allowed for the preservation of beta (β) diversity in the gut microbiota, whereas the placebo group saw a decrease in this metric [52][86]. Rifaximin demonstrates great potential for maintaining the balance between the host and the gut microbiota. Molecular and microbiological data are strongly consistent with clinical evidence. Rifaximin’s effectiveness, however, may be impacted by various disruptions to the gut homeostasis that are related to various pathological conditions. On the other hand, rifaximin is a versatile drug because it has the ability to restore balance instead of acting on a single target. Regarding one of the main issues with antibiotic therapy, rifaximin showed no long-term effects, and its impact on bacterial resistance appears to be absolutely negligible [41][75]. The eubiotic effects of rifaximin are summarized in Figure 2.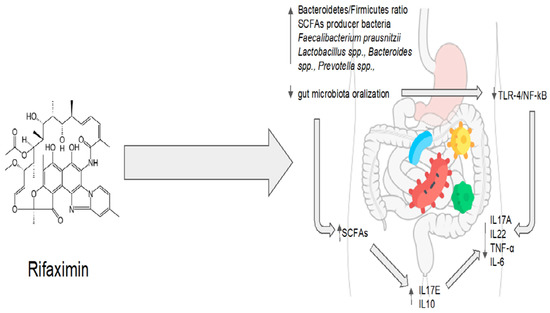
Figure 2. Rifaximin’s eubiotic effects. IL17A: interleukin 17A; IL22: interleukin 22; TNF-α: tumor necrosis factor alpha; IL-6: interleukin 6; IL10: interleukin 10; IL17E: interleukin 17E.
2.3. Other Antimicrobial Agents: Triclosan
Triclosan is a nonionic broad-spectrum antimicrobial agent. Its chemical name is 2,4,4′-trichloro-2′-hydroxydiphenyl ether. It is widely used in toothpaste, food storage containers, medical products, personal care products, and plastic cutting boards [56][90]. Triclosan acts as a detergent, directly disrupting the integrity of the bacterial membrane [57][91]. Moreover, it interferes with the synthesis of bacterial fatty acids by inhibiting the enoyl-acyl carrier protein (enoyl-ACP) reductase [58][92]. At low concentrations, it limits bacterial growth, while at high concentrations, it is bactericidal [59][93]. Since its introduction, numerous studies have examined the effects of triclosan on living organisms to determine its safety. A randomized study of triclosan-containing household and personal care products carried out to evaluate the safety of triclosan showed that triclosan exposure did not induce global reconstruction or the loss of microbial diversity in the gut microbiota [60][94]. Nevertheless, because triclosan-containing products included toothpaste, the oral microbiota was also examined. So far, even in sites where triclosan was applied directly, no significant differences in α-diversity were detected [60][94]. Triclosan seems to not have an antibacterial effect at the dosage used in household and personal care products, despite the fact that this effect may be present at higher concentrations and for longer periods of exposure [60][61][94,95]. Another preclinical model sought to define perturbations to the gut microbiota caused by triclosan. Microbiota composition was evaluated at three, twenty-one, and fifty-two weeks after the administration of a low dose of triclosan. Following exposure to triclosan (50 mg/kg/day), the abundance of Bacteroidetes increased at week 52. At the same dose after 52 weeks, triclosan slightly (but not significantly) reduced the abundance of Firmicutes. At 21 and 52 weeks of age, triclosan decreased the levels of Akkermansia muciniphila at the species level. Low doses of triclosan increased α-diversity after three weeks when compared to the control group [62][96]. Triclosan resistance mechanisms include changes in the enoyl-ACP reductase and efflux pumps. However, despite the widespread use of products containing triclosan, the community’s overall resistance and cross-resistance rates are low [63][97]. Triclosan consequently gained attention as a modulator of the gut microbiota, and it was considered as a drug for the treatment of dysbiosis in a recent preclinical study [64][98]. Mice received a continuous high-fat diet for 20 weeks and were then treated with triclosan at a dose of 400 mg/kg/d for the final 8 weeks [64][98]. In mice fed a high-fat diet, triclosan increased the ratio of Bacteroidetes/Firmicutes and decreased the number of pathogenic Gram-negative bacteria, such as Helicobacter, Erysipelatoclostridium, and Citrobacter, as shown by a metagenomic analysis [64][98]. Triclosan also increased the relative abundance of Lactobacillus, Bifidobacterium, and Lachnospiraceae, which protect against abnormal metabolic processes [64][98]. The increase in α- and β-diversity also demonstrated triclosan’s improvement of bacterial diversity and richness [64][98]. On the other hand, a cohort study showed that triclosan exposure causes a decrease in the diversity of the gut microbiota in breast-fed infants [65][99]. This study, however, is not representative of the general population due to the small sample size and the high vulnerability of the infant gut microbiota to antimicrobial agents. Additionally, rather than thinking of triclosan as a perturbator of a balanced microenvironment, it would be interesting to consider it as a modulator of the gut microbiota in patients with a state of gut dysbiosis [65][99]. Indeed, triclosan is employed in periodontal disease, which could be considered a model of oral dysbiosis [66][100]. Nonetheless, oral dysbiosis, which is characterized by an increased abundance of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans, has been associated with the impairment of the gut microbiota equilibrium and the loss of gut microbial diversity [67][68][101,102]. Moreover, these oral pathobionts have been associated with NAFLD and NASH progression in preclinical models [67][69][101,103]. As a consequence, periodontal therapy can improve oral and gut dysbiosis in patients with chronic liver diseases [70][104]. In particular, periodontal therapy in cirrhotic individuals reduces blood levels of inflammatory cytokines and lipopolysaccharide (LPS), with beneficial effects upon both quality of life and in mitigating the development of hepatic encephalopathy [70][104]. Triclosan, as a treatment for periodontal disease [71][105], has been shown to reduce the production of pro-inflammatory cytokines (such as IL-8, IL-1 α, and TNFα) in a human epithelial cells, monocytes, and fibroblast cultures exposed to LPS [72][106]. More specifically, triclosan treatment inhibits the TLR-4 pathway by inducing the microRNA miR146a to downregulate IRAK1 and TRAF6 proteins. Conversely, triclosan exposure increases the epithelial cells’ production of other bioactive anti-microbial molecules, such as β-dsefensins [72][106]. Interestingly, β-defensins have been proposed as a key factor in the physiological homeostasis between the host and the microbiome [73][107]. In conclusion, triclosan, an antimicrobial agent with a long history, has been suggested to have a positive impact on the microbiota in the human gut. In addition to its well-known antibacterial action, triclosan could have a pleiotropic effect on different cell types, directly orchestrating physiological homeostasis between microbiota and their host. However, more investigations are necessary. Triclosan’s eubiotic effects are summarized in Figure 3.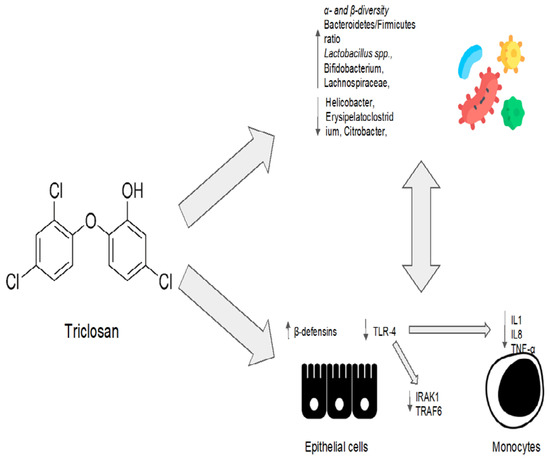
Figure 3. Triclosan’s eubiotic effects. IL1: interleukin 1; IL8: interleukin 8; TNF-α: tumor necrosis factor alpha; IRAK1: interleukin 1 receptor-associated kinase 1; TRAF6: TNF-receptor-associated factor 6.
2.4. Natural Products: Promising Agents for the Modulation of Microbiota
Evodiamine is an alkaloid, mainly present in the Evodia Fructus, which is officially listed in the Chinese Pharmacopoeia as a remedy for patients suffering from viral hepatitis, cholangitis, and gastric ulcers, among other disorders [74][108]. Evodiamine has been demonstrated to have an antimicrobial activity in vitro, inhibiting bacterial topoisomerase I [74][108]. Evodiamine’s impact on intestinal inflammation and the gut microbiota was examined in a preclinical study [75][109]. In order to create a intestinal inflammatory tumor mouse model, azomethane/sodium dextran sulfate was used [75][109]. The tumor model was then treated with evodiamine and 5-aminosalicylic acid. Evodiamine and 5-aminosalicylic acid both prevented the growth of tumors and induced the death of tumor cells [75][109]. A quantitative polymerase chain reaction analysis showed that evodiamine and 5-aminosalicylic acid reduced the number of Enterococcus faecalis and Escherichia coli while increasing the abundance of Bifidobacterium, Campylobacter, and Lactobacillus when compared to the control group [75][109]. The IL6/STAT3/P65 signaling pathway was inhibited, and levels of inflammatory factor, d-lactic acid, and serum endotoxin were all significantly decreased in the evodiamine group [75][109]. Evodiamine also showed efficacy in preventing colorectal tumors in a mouse model of chemically induced colitis [76][110]. In this model, evodiamine increased the abundance of SCFA-producing bacteria, inhibiting the harmful bacteria [76][110]. After evodiamine treatment, histological analysis showed a remarkable reversion of intestinal epithelial structure destruction, inflammatory cells infiltration, and crypt loss. The intestinal barrier was also restored, with an increased expression of occludin, zonula occludens-1, and E-cadherin [76][77][110,111]. Additionally, evodiamine decreased the expression of pro-inflammatory genes involved in the Wnt signaling pathway, the Hippo signaling pathway, and the IL-17 signaling pathway [76][110]. This anti-inflammatory effect could be mediated by the downregulation of the nuclear factor-kappa B (NF-κB) signal and the inhibition of NLRP3 inflammasome activation, as shown in another sodium-dextran-sulfate-induced colitis murine model [77][111]. The effect of evodiamine was also evaluated on H. pylori in an in vitro gastric adenocarcinoma model [78][112]. Evodiamine treatment decreased the bacterial production of the type IV secretion system components and the system subunit protein A protein and the expression of cytotoxin-associated antigen A (CagA) and vacuolating cytotoxin A (VacA). This resulted in a reduction in CagA end VacA proteins in tumor cells. In addition, evodiamine specifically prevented the H. pylori-infection-induced stimulation of signaling proteins such as NF-κB and the mitogen-activated protein kinase (MAPK) pathway. As a result, IL-8 secretion in tumoral cells was reduced [78][112]. Evodiamine and berberine were combined in order to determine their effect on the gut microbiota of rats fed a high-fat diet [79][113]. The treatment with evodiamine and berberine increased the diversity of the gut microbiota globally [79][113]. The gut microbiota profiles were determined via the high-throughput sequencing of the bacterial 16S ribosomal RNA gene [79][113]. In comparison to the control group, there were higher proportions of SCFA-producing bacteria (Lactobacillus, Prevotella, Ruminococcaceae, and Bacteroides). In contrast, the key bacteria responsible for the imbalance in the gut microbiota in the group with a high-fat diet were Fusobacteria and Lachnospiraceae [79][113]. The high-fat-diet group’s Firmicutes/Bacteroidetes ratio was significantly higher than that of the control group [79][113]. The administration of evodiamine and berberine reverted the increasing trend of the ratio. Evodiamine–berberine also lowered the intestinal submucosal edema typical of a high-fat diet and the mucosal inflammatory cell infiltration [79][113]. As a result, the rats treated with evodiamine and berberine showed a significant reduction in body weight, as well as plasma triglyceride and total cholesterol levels [79][113]. Liver injury (measured via plasma levels of aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transpeptidase) was significantly reduced [79][113]. Evodiamine’s eubiotic effects are summarized in Figure 4.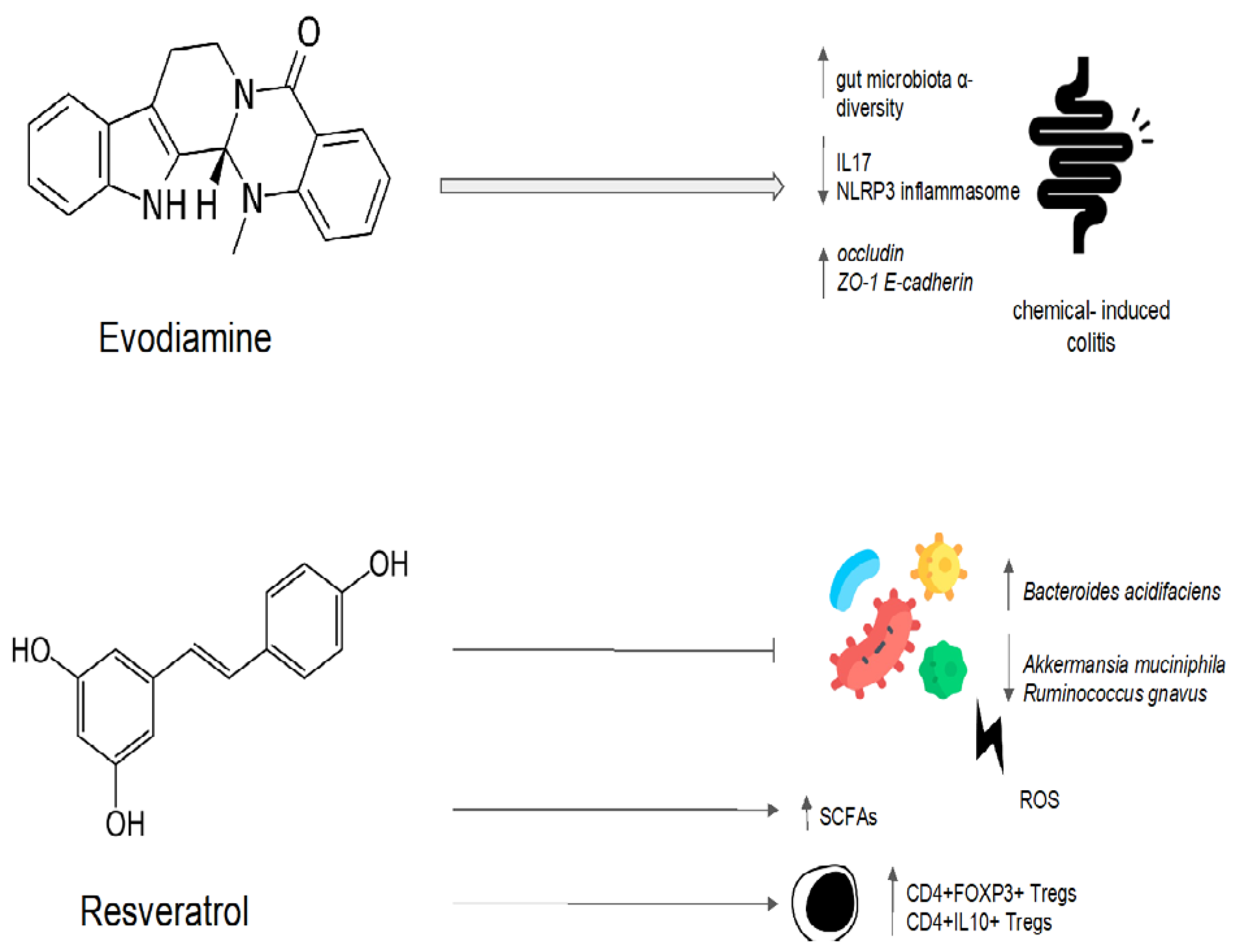
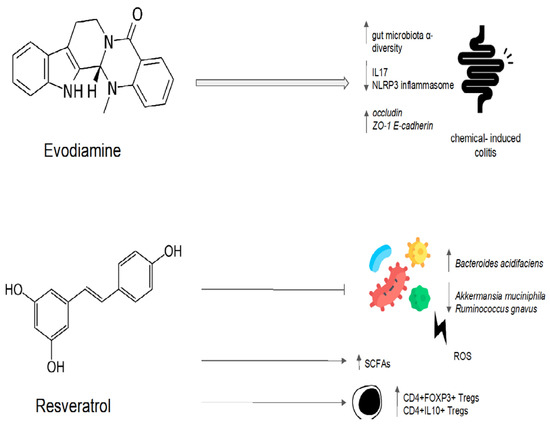
Figure 4. Possible mechanisms of the eubiotic effect of evodiamine and resveratrol. IL17: interleuchina-17; NLRP3: NOD-like receptor family, pyrin domain-containing protein 3; ROS: reactive oxygen species; ZO-1: zonula occludens-1; E-cadherin: epithelial cadherin.
