Fluorinated greenhouse gases (F-gases) are used for various applications, such as in refrigeration and air conditioning, as substitutes of the ozone-depleting substances. Their utilization has increased drastically over the last few decades, with serious consequences for global warming. The Kigali Amendment to the Montreal Protocol and several national and international legislations, such as the 2014 EU F-gas Regulation, aim to control the utilization and emissions of these gases. In the EU, the phase-down of hydrofluorocarbons (HFCs) is underway, with successive reductions in quotas up to 2050. Under this scenario, efficient strategies for managing the produced and already existing F-gases are of vital importance to guarantee that their effect on the environment is mitigated. Up to now, most of the F-gases recovered from end-of-life equipment or when retrofitting systems are either released into the atmosphere or destroyed. However, in order to put forward a cost-efficient adaptation to the F-gas phase-down, increasing separation and recycling efforts must be made.
1. Introduction
Since the 19th century, several gases with different chemical and physical properties have been investigated for refrigeration and other industrial applications. From the first toxic and flammable compounds, great advances have been made until the development of the refrigerants used nowadays. In the 20th century, chlorofluorocarbons (CFCs) gained great popularity due to their non-toxicity, non-flammability, and high efficiency, and therefore their atmospheric concentrations increased drastically. However, it was found that these compounds are not harmless and destroy the ozone layer
[1]. In this scenario, compounds lacking chlorine atoms, such as hydrofluorocarbons (HFCs), emerged as substitutes for ozone-depleting compounds
[2]. HFCs, together with perfluorocarbons (PFCs), sulfur hexafluoride (SF
6), and nitrogen trifluoride (NF
3), are the so-called fluorinated greenhouse gases (F-gases). These synthetic gases have several advantages over naturally occurring gases, such as durability, inertness, non-flammability, non-explosivity, non-reactivity, non-toxicity, and thermal stability
[3]. While these properties are ideal for refrigeration and other industrial applications, these compounds are harmful to the environment. Most F-gases are highly stable in the atmosphere, with an atmospheric lifetime of up to 50,000 years, and are potent greenhouse gases (GHGs), with a global warming potential (GWP) up to 23,000 times greater than that of CO
2. Consequently, small atmospheric concentrations of F-gases induce large effects on global temperature and have a great impact on climate change. Upon releasing, F-gases persist in the atmosphere and their effects cannot be controlled, affecting the environment for long periods. Therefore, the substitution of F-gases by other compounds is essential to control their impacts in the environment.
In 2015, F-gases represented 2% of the global GHG emissions
[4], and in 2018 they were 3% of the GHG emissions in the EU
[5]. Considering the high GWP of the F-gases, they have a major contribution to global warming. While the emissions of all other GHGs in the EU have decreased, the emissions of F-gases almost doubled from 1990 to 2014
[5] and were expected to have a 4 to 5-fold increase in 2005–2050 if efficient measures were not taken
[6].
A key aspect of F-gases is that they are synthetic. Therefore, unlike natural pollutants, their production, use, and emissions can be almost totally controlled if appropriate and efficient legislations and practices are applied. The transition into alternatives with lower GWP is already underway and it is expected that it can be achieved almost totally in the near future. However, most of the F-gases produced in the last few decades are stored inside equipment or are part of several products. Therefore, the correct handling and management of these equipment and products, even after the complete transition of the new alternatives, is key to control the release of F-gases into the atmosphere.
2. Applications
F-gases are widely used for several industrial and commercial applications, mainly as refrigerants in cooling systems, as blowing agents in the manufacturing of organic and inorganic foams and materials, in the manufacturing of electronics, and as part of aerosols, fire extinguishers, and solvents. The most important HFCs used today include the HFC R-134a (GWP 1430) and the HFC blends R-404A (GWP 3922) and R-410A (GWP 2088)
[7]. R-404A (GWP 3922) is a blend of R-125 (GWP 3170), R-134a (GWP 1430) and R-143a (GWP 4800), and R-410A (GWP 2088) is a blend of R-32 (GWP 677) and R-125 (GWP 3170).
Almost half of F-gas emissions and about 86% of the HFC consumption (in GWP) are due to cooling and refrigeration systems
[8]. The dependence on these systems, and therefore on F-gases, is increasing worldwide since societies depend largely on: (i) industrial and commercial refrigeration for the functioning of food, chemical, pharmaceutical and other industries, and of the retail sector (for example, supermarkets and restaurants); (ii) domestic refrigerators and freezers in houses and offices; (iii) refrigerator containers for transport; and (iv) stationary and mobile air conditioning to cool personal and commercial transportation vehicles, houses, offices and other buildings. Most systems rely on high GWP F-gases, such as the CFC R-12 (GWP 10,200), the HFC R-134a (GWP 1430), and the HFC blends R-404A (GWP 3922) and R-507A (GWP 3985), and have high leakage rates
[9]. These direct emissions of GHGs, together with the high electricity demands (almost 17% of global electricity use) that represent indirect GHG emissions, are responsible for making the cooling and refrigeration sector one of the main contributors to global warming
[9].
Today, almost 4 billion pieces of cooling equipment are estimated to be in use globally. This number may rise up to 14 billion by 2050 as a consequence of the rise in global temperatures and of the increasing capacity of the populations in developing countries to have access to cooling equipment
[9]. The global energy demand for building cooling more than tripled between 1990 and 2016 and the installed capacity is predicted to rise from 11,670 GW in 2016 to over 36,500 GW in 2050
[9]. Without the international agreements and the application of legislation at international and national levels, the direct and indirect emissions from the air conditioning and refrigeration sectors are projected to rise at least 90% from 2017 levels by 2050
[9][10]. The increased utilization of F-gases for cooling leads to global warming, creating a feedback loop with dangerous consequences.
Buildings last for several decades and therefore changes in the climate (i.e., increase in temperature and changes in other factors such as wind, solar radiation, and humidity) greatly influence their energy profile over the years. Several studies have addressed the impact of climate change on buildings’ energy use, using different methods and climate models
[11][12][13][14][15][16]. While the impacts will be different according to the geographic location, for all regions a decrease in energy use for heating and an increase in energy use for cooling are expected. Since the dependence on refrigeration and cooling will continue to increase in the foreseeable future, it is vital to switch to low GWP refrigerants.
In emergent economies, the increasing demand for air conditioning systems is not being accompanied by the utilization of environmentally friendly alternatives, and high GWP and ozone-depleting refrigerants, such as R-22 (GWP 1760), still dominate the markets
[17]. In the markets where R-22 (GWP 1760) is being phased out, the high GWP R-410A (GWP 2088) is the most used refrigerant
[17]. The refrigerant R-410A (GWP 2088) is currently being phased out under the current EU legislation and the transition to new more environmentally friendly alternatives is taking place.
The market of the air conditioning systems in cars and other transportation vehicles is expanding largely. Despite containing smaller amounts of refrigerant in comparison to other refrigeration and cooling equipment, the leakage rates of mobile air conditioning systems are much higher
[3]. Most equipment uses R-134a (GWP 1430), which was adopted worldwide as a substitute to the CFC R-12 (GWP 10,200), and a transition to the low GWP unsaturated HFC R-1234yf (GWP 4) is being put forward
[17]. However, the high leakage rates, the inadequate refrigerant handling during maintenance, and improper disposal or release of F-gases from equipment containing high GWP refrigerants still make these equipment major contributors to global warming
[3]. In fact, since the treatment of the recovered F-gases has associated costs, the illegal release of the F-gases from end-of-life (EoL) equipment is, in most cases, chosen.
Paradoxically, cooling systems, which have been improving the well-being of societies, are endangering them. As has been pointed out in a study focused on evaluating the efficiencies of the existing room air conditioners in developed countries and in emerging markets, there are still many improvements to make in this sector
[17]. In this way, it is essential to improve the energy efficiency of the equipment during the transition to low GWP refrigerants, and to reduce energy consumption, environmental impacts, and costs. Moreover, new technologies and waste electrical and electronic equipment (WEEE) and end-of-life vehicles (ELV) management processes must be implemented in the refrigeration and air conditioning sector.
3. Impacts
The rise in the emissions of F-gases and other GHGs drives global warming and consequently leads to the rise in the sea level and to more frequent and severe floods, droughts, and wildfires. This has severe impacts not only on the health and well-being of populations but also on all earth ecosystems. The impacts of climate change on biodiversity include habitat loss and subsequent invasion of foreign species (including humans), disruptions in food chains, and changes in reproduction cycles
[18]. Moreover, upon releasing into the atmosphere, F-gases are degraded into harmful by-products, such as hydrogen fluoride (HF) and trifluoroacetic acid (TFA). These compounds can also be generated in destruction facilities upon the incineration of F-gases, and if no appropriate treatment is applied, they are released into the atmosphere. In the atmosphere, both HF and TFA are dissolved in water and are directed to the earth’s surface by precipitation, leading to water acidification, and possibly to a significant eco-toxicity upon accumulation in the ecosystems
[19]. The drastic climate changes induced by human actions in the last century increased the pressure on the ecosystems to levels impossible to be balanced by natural mechanisms, leading to a biodiversity loss with the fastest rate experienced in the last 65 million years.
HFCs started to be massively used after the implementation of the Montreal Protocol as an alternative to substances that deplete the ozone layer. However, recent studies have demonstrated that despite the indirect effects of HFCs on ozone depletion being small, they are noticeable, considering the great accumulation of these substances in the atmosphere
[20]. The increase in the temperature of the troposphere and stratosphere, as a result of the accumulation of HFCs, accelerates the catalytic destruction of ozone. According to that study, HFCs may cause a 0.035% decrease in the ozone layer by 2015, with R-125 (GWP 3170) accounting for approximately 50% of the changes in global temperature and in the ozone levels
[20].
HFCs are, therefore, a clear example of a group of substances whose use was promoted with the reasoning of being greener and environmentally friendly, but which revealed unexpected effects, prompting the implementation of new international legislations and seeking new alternatives. While the release of other GHGs may have natural causes, HFCs and other F-gases are synthetic compounds, and their release is strictly related to human actions. In this way, the reduction in the effects of F-gases depends on the establishment of efficient legislation and practices to seek new alternatives and to manage the existing F-gases.
4. WEEE and ELV Management and Circular Economy
A multi-way approach to mitigate the effects of F-gases on the climate must include: (i) the phase-down of high GWP compounds and seeking new alternatives; (ii) the improvement of equipment to prevent leaks and the promotion of good handling and operation practices to prevent emissions; and (iii) the efficient management of the produced F-gases, of those recovered from EoL equipment, and of those recovered when retrofitting existing installations. According to the EU F-Gas Regulation, the release of refrigerants into the atmosphere is explicitly prohibited and subjected to penalties. Therefore, refrigerants must be recovered for reuse or destruction
[21].
However, much improvement still needs to be achieved globally in the recovery and management of F-gases and other refrigerants. There are two major sources of F-gas residues, WEEE and ELVs, which require different management approaches. Despite having legislation that regulates these procedures and having economic capacity to implement efficient (and sometimes expensive) technologies, developed countries still face challenges to efficiently manage refrigerants. In Portugal, data from 2017 revealed that only 8.7% of the refrigerants were recovered from equipment and systems and further treated, most probably due to incorrect management by non-certified operators
[22]. In developing countries and emerging economies, refrigerant management is even more challenging, mostly due to the lack of efficient legislation, difficulties in implementing technologies, and to the dismantling of equipment to recover metals and other material with no concerns for the potential refrigerant release.
The emission of refrigerants from air conditioning systems in vehicles is challenging due to the high leakage rates in the systems, to the unintentional release of refrigerants due to car accidents, and to the improper recovery and management during the dismantling process. In the EU, the directive 2000/53/EC requires Member States to ensure that EOL vehicles are dismantled and hazardous materials, including HFC refrigerants, are removed
[23]. According to Directive 2006/40/EC, from 2017 onward the air conditioning systems of all new vehicles cannot contain F-gases with a GWP higher than 150 (e.g., R-134a)
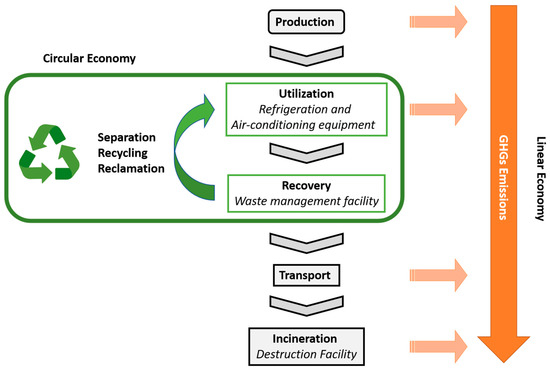
[24]. Despite the legislation that determines the switch to lower GWP alternatives in vehicles, the mechanisms to manage the F-gases from EoL equipment present flaws. To reduce the release of F-gases into the atmosphere, they must be recovered from EoL equipment for further destruction or reuse. However, since most workers are not well trained, few incentives are given to the recovery and disposal processes, and to the obligation to pay the costs of disposing, most operators responsible for recovering the refrigerants end up releasing them into the atmosphere. Moreover, a great amount of refrigerant is lost during the system’s lifetime. Upon recovery, F-gases can be managed in two main ways. They can be destroyed or they can be reprocessed for further reuse ()
[25]. The first approach follows a linear economy concept and has the advantage of using already implemented technologies and facilities to achieve an almost complete elimination of the F-gases
[25][26]. However, emissions of GHGs occur during the transport of the F-gases from the site where they were recovered to the incineration facility and during the incineration process. The second approach follows a circular economy concept and has the advantage of allowing the reuse of value-added refrigerants to reduce the pressure on the supply chain of these compounds, reducing the release of GHGs during the production of new compounds and during their destruction
[25]. Moreover, if the reprocessing of the F-gases occurs in the same place where they were recovered, additional benefits in terms of costs and GHG emissions are obtained since the transport (sometimes over long distances) is avoided.
Figure 1. Possible approaches for the management of F-gases, following either a linear economy concept (from production to incineration) or a circular economy concept (involving separation, recycling and/or reclamation processes).
4.1. Recovery and Destruction
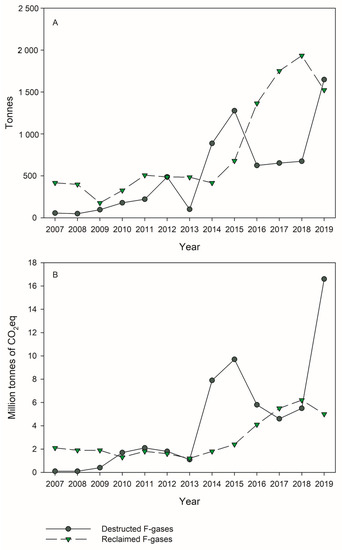
The destruction of refrigerants, including HFCs and ozone-depleting substances such as CFCs and HCFCs, is considered the best practice for the long-term goal of eliminating these compounds, promoting the switch to more environmentally friendly alternatives. The prohibitions in the use of some HFCs and the phase-down of these compounds only started in the last decade, and therefore little data are available regarding their destruction. This contrasts with ozone-depleting substances, which were prohibited starting in the 1990s and whose destruction is still being performed due to the great amounts of compounds present in equipment and products. In the EU, the reported values of destroyed F-gases increased significantly in 2019 compared to 2018 (). This increase was of 145% considering the amount of F-gases (to a total of 1648 tons in 2019) and of 200% considering the CO
2eq (to a total of 16.6 million tons CO
2eq in 2019) ()
[27]. Almost the totality of the reported destroyed F-gases in 2019 referred to HFCs. In the same year, 2019, around 1753 tons of CFCs and HCFCs were reported as destroyed in the EU
[28].
Figure 2. Destruction and Reclamation of F-gases in the EU (2007–2019) according to the EEA Report No 15/2020 on fluorinated greenhouse gases
[27]. Data in tonnes of gas (Panel (
A)) and in million tonnes of CO
2eq (Panel (
B)).
According to an ICF study prepared for the European Commission
[29], at least 25 facilities in the EU-27 performed commercial destruction of HFCs and ozone-depleting substances. Fourteen of the identified facilities had an estimated combined destruction capacity of approximately 130,000 tons per year
[30]. While some countries have more than one facility (e.g., Germany with eight facilities and Hungary with four facilities), others did not have any facilities at that date (e.g., Portugal and Italy)
[30]. The costs for the destruction of HFCs and ozone-depleting substances in the EU have been estimated in the range of EUR 1–EUR 10 per kg
[30]. Since most destruction facilities are located away from the place where the gases are recovered and collected, and in many cases are not even located in the same country, the additional costs of recovery and transportation (estimated to be EUR 0.10 per tonne per kilometer in the EU) must be added
[30].
The applicability of current destruction technologies, previously approved for ozone-depleting substances
[31], to HFCs has been assessed in 2018 by the UNEP Technology and Economic Assessment Panel Task Force on Destruction Technologies
[26]. The recommendations include specifications for: (i) a minimum Destruction and Removal Efficiency (DRE) of 99.99% for concentrated sources or 95% for dilute sources; (ii) emissions of dioxins and furans, HCl, chlorine, HF, HBr, bromine, particulate matter, and CO; and (iii) technical capability to be applied at a commercial scale. Since corrosive and harmful products, such as HF and HCl, are generated during the destruction of HFCs, HCFCs, and CFCs, the systems must be resistant to these compounds and should integrate their neutralization in the process. In many processes, to reduce the generation of those by-products, only small amounts of HFCs and ozone-depleting substances can be treated or are treated together with other compounds.
Three types of technologies that have been used for the destruction of ozone-depleting substances are being used (or being evaluated) for the destruction of HFCs: thermal oxidation (incineration) technologies, plasma technologies, and non-incineration technologies involving chemical transformations
[26]. Destruction technologies are expensive, with high initial investments and high operation and energetic costs
[26]. Globally, incineration technologies are by far the most used ones due to the lower costs and easiness of implementation (since some facilities are also used to destruct other substances) compared to the other available technologies
[26].
4.1.1. Incineration
Incineration at temperatures over 1000 °C has been widely used for the destruction of ozone-depleting substances, such as CFCs and HCFCs. An advantage of incineration is that the generated energy can be harnessed for other processes. It has also been used for the incineration of the HFC R-23 (GWP 12,400), a high GWP compound that is a by-product of the manufacturing of the HCFC R-22 (GWP 1760), which is used as a refrigerant and in blends in foam blowing. Despite being in a phase-out process, the production of R-22 (GWP 1760) has increased, mostly due to its production in developing countries for direct use and due to its worldwide production as a component to make other products. Incineration has been used for the destruction of R-23 (GWP 12,400), but a recent study showed that the atmospheric concentrations of this gas reached very high levels in 2018, beyond what was projected considering the reported R-22 (GWP 1760) production
[32]. This suggests that the R-23 (GWP 12,400) incineration facilities, mainly in developing countries, have been deactivated or are not working properly.
The incineration technologies that were developed for the incineration of ozone-depleting substances (either in a dedicated process or in co-incineration with other waste) and of R-23 (GWP 12.400) have been used in some facilities for the degradation of other HFCs. The thermal stability of fluorinated compounds, such as HFCs, has been compared to the one of chlorinated compounds and it was concluded that fluorinated compounds can be destroyed with high efficiency by incineration
[33]. The technologies currently in use include cement kilns, liquid injection incineration, municipal solid waste incineration, reactor cracking, and rotary kiln incineration, which have also been used with high potential for the destruction of HFCs, including R-23 (GWP 12,400), R-32 (GWP 677), R-125 (GWP 3170), R-134a (GWP 1300), and R-143a (GWP 4800)
[26]. The technologies of gaseous/fume oxidation and porous thermal reactor have been approved for the destruction of HFCs since they were demonstrated to meet the performance criteria established by the UNEP TEAP Task Force on Destruction Technologies
[26]. Moreover, alternative methods have been studied at pilot- and full-scale with high efficiency and low emissions. These include a gasification melting system in reductive conditions
[34].
Despite being effective for the destruction of HFCs and ozone-depleting substances, incineration processes contribute to global warming due to the release of CO2, and generate harmful by-products. In this way, these processes need to be efficiently designed and operated and tightly controlled to keep emissions at minimum levels.
4.1.2. Plasma Technology
Plasma technologies, based on the destruction of the compounds by the heat generated by a plasma arc, are currently being used to destroy ozone-depleting substances, and HFCs in some cases. The gaseous by-products of these processes include HCl, HF, CO, and CO2. These technologies provide high destruction efficiencies, fast decomposition with large throughput, quick start-up and shut-down, greater flexibility of operation and low gas emissions. Moreover, the systems are designed to prevent the formation of dioxins and furans and to neutralize the acids. However, they have high energy consumption.
Different technologies have been developed differing on the methods for producing plasma: by radio frequency, by microwave energy or by an electric plasma torch in the presence of argon or nitrogen. The most widely used technology is the one using argon gas, in facilities operating commercially worldwide. Utilization for the destruction of HFCs and HFC/HCFC blends was reported in Australia, the United States and Mexico. Technology using nitrogen gas was reported for the destruction of R-23 (GWP 12.400) in China and of the HFC R-134a (GWP 1300) and of the HFC blends R-410A (GWP 2088) and R-407C (GWP 1774) in Japan. The destruction of R-134 (GWP 1300) using microwave plasma has been investigated and a total conversion degree up to 84%, with no interconversion into other HFCs, was observed
[35].
Besides these technologies, others are being investigated and developed specifically for HFCs, aiming to increase efficiency, to improve the flexibility of operation, and to decrease costs of implementation and operation. Such technologies include a water plasma
[36] and a dielectric-packed-bed nonthermal plasma reactor with barium titanate beads as the packing material
[37], for the decomposition of R-134 (GWP 1300).
4.1.3. Other Technologies
The destruction of ozone-depleting substances can also be achieved by converting them into other useful by-products, instead of incinerating them. Different technologies have been approved for the destruction of those substances and have shown potential to also be used for the destruction of HFCs. These technologies include: (i) the conversion by hydration of the compounds into HF, HCl, and CO
2 in a superheated steam reactor whose walls are heated up to 1000 °C, followed by quenching and neutralization of the acids using a Ca(OH)
2 solution
[26]. This technology, which is being applied in commercial facilities in Japan and China, is safe, easy to operate and has high destruction efficiency and low emissions of pollutants
[26]; (ii) the Gas Phase Catalytic Dehalogenation of the compounds at 400 °C with the neutralization of produced HCl and HF in a Ca(OH)
2 solution
[26]. Japan reported the utilization of this technology to destroy CFCs, HCFCs and HFCs or HFC blends, such as R-134a (GWP 1300), R-245fa (GWP 858), R-404A (GWP 3922), R-407C (GWP 1774), R-407D (GWP 1627), R-407E (GWP 1552), R-410A (GWP 2088), R-417A (GWP 2346), R-507A (GWP 3985) and R-508A (GWP 13,214)
[26]; and (iii) the conversion of the compounds into HF, HCl, CO, and H
2O by chemical reaction with H
2 and CO
2 [26]. The produced acids are not neutralized but collected as high purity products
[26]. This technology is used by a company in the United States and has been tested for the degradation of HFCs in China
[26].
Other technologies have been investigated for the degradation of HFCs, including the photodegradation of R-134a (GWP 1300)
[38] and R-152 (GWP 16)
[39] with TiO
2 and UV light, and the degradation of HFCs using Bi
2O
3 as a photocatalyst under visible light irradiation
[40].
4.2. Reuse of HFCs: Circular Economy
Despite being efficient in eliminating F-gases and fully or partially reducing their harmful effects on the environment, destruction processes may contribute to the release of GHGs. The quota systems imposed by the current F-gas legislations increased the pressure on the HFC supply chain and led to a rise in the prices of virgin refrigerants newly placed on the market. In this context, in some cases the reusing of valuable refrigerants recovered from EoL equipment has clear advantages over their destruction ().
Moreover, destruction facilities are not located close to most industries and waste management facilities and do not exist in every country. Consequently, great amounts of F-gases are transported through long distances, drastically increasing costs (estimated EUR 0.10 per ton per kilometer in the EU
[30]), the environmental impact (average emission value of 62 gCO
2 per tonne of transported cargo per km
[41]), and the chances of unintentional release of the transported gases. On the contrary, it is easier and more feasible to install processing and recycling facilities close or on the same site where the F-gases are recovered ().
A quick and cheap way of reusing refrigerants consists of cleaning-up the old refrigerant and feeding it to the same system in which it has been used before. This approach, frequently named recycling, does not guarantee that the cleaned-up refrigerant conforms to the standards of new refrigerants
[30]. This may be particularly problematic in the case of complex blends, which may contain HFCs, HFOs, and other gases. In systems presenting considerable leaks, the composition of the blends can change over time due to differences in the boiling points of their constituents. Thus, the simple reutilization of the refrigerant may be problematic in terms of performance, energy use, system maintenance, and costs, due to changes in the refrigerant composition and to the presence of impurities.
A more advantageous approach is to use reclaimed refrigerants, defined by the EU as the ones that were recovered from EoL equipment and were reprocessed to meet the parameters of virgin substances, considering its intended use
[42]. The reclaimed F-gases need to meet the requirements of the AHRI Standard 700 (2019) for their reuse, which are: (i) a purity of ≥ 98%, (ii) presence in the vapor phase of less than 1.5% by volume (at 25 °C) of non-condensable impurities, and (iii) presence in the liquid phase of less than 10 ppm by weight of water, less than 0.5% by weight of all other volatile impurities, less than 0.01% by volume or weight of high boiling residue, less than 1 ppm by weight of acidity (as HCl), and non-visually detectable presence of particles/solids and chloride
[43].
Under the EU F-gas regulations, only virgin refrigerants (not reclaimed) that are placed on the market are included in the quotas. Thus, the utilization of reclaimed HFCs contributes to reducing the pressure on the virgin HFC supply chain, reducing the industry’s dependence on costly high GWP refrigerants, and achieving the HFC phase-down goals (). Up until now, virgin refrigerants were freely available at a low cost and therefore reusing refrigerants was not often considered. However, in the EU the first significant reduction in the quota took place in 2018 and the next one will occur in 2021. Therefore, the industry’s ability to reduce, reuse, and innovate is vital for the transition to a restricted market. As HFC phase-down progresses, the utilization of reclaimed F-gases is increasing. According to the 2020 EEA report on fluorinated greenhouse gases, the reported reclaimed HFCs now make up 8% (9% as CO
2eq) of the produced amount or 3% (4% as CO
2eq) of the EU supply of virgin HFCs ()
[18]. While 97% of reclaimed amounts are HFCs, SF
6 contributed to about 20% (GWP) of reclaimed gas
[27]. However, there is no obligation in the EU to report the reclaimed F-gases, which can make it harder to evaluate the current situation. In fact, in 2019 a strong decline in the reported reclaimed F-gases occurred, which was explained by incomplete reporting
[27].
To increase the reuse rates, efforts need to be made to develop the recycling and reclamation infrastructures and to improve legislation to allow an efficient and tight control of the transport of reclaimed refrigerants. In Europe, there are already manufacturers that recover refrigerants, such as R-410A (GWP 2088), process them to virgin specifications and then sell them across Europe
[44].
4.3. New Technologies for the Recovery and Separation of HFCs
Despite the benefits of reclaiming refrigerants at short term, at long term the switch to alternatives with lower GWP must be made. However, some of the high GWP blends currently in use are still valuable. The constituents of the most used high GWP blends, such as R-410A (GWP 2088) and R-404A (GWP 3922), have high value compounds that can be used as single component refrigerants or in low GWP blends.
The blend R-410A (GWP 2088) is one of the main refrigerants used in refrigeration and air conditioning systems. However, due to its high GWP, it is being substituted by alternatives with lower GWP. One of those alternatives is R-32 (GWP 677), one of the components of the blend together with R-125 (GWP 3170), mostly in low-capacity systems. This refrigerant was present in approximately 37% of all split systems in 2019 and is expected to exceed 80% by 2023
[45]. In Japan, a complete shift towards R-32 (GWP 677) occurred in produced residential split air conditioners, starting from 2014
[46]. In some air conditioning sectors, blends of R-32 (GWP 677) with other HFCs and HFOs, such as R-454B (GWP 466) and R-452B (GWP 676), are being promoted as alternatives to R-410A (GWP 2 088).
Therefore, there are undeniable advantages in separating value-added F-gases from blends recovered from EoL equipment and further recycling and reusing them, either as single component refrigerants or in blends. However, this approach has received little encouragement and no developed technologies are currently available. Since most refrigerants are azeotropic or near-azeotropic mixtures, conventional processes, such as distillation, are not efficient or feasible. For example, R-410A (GWP 2088) is a near-azeotropic system of R-32 (GWP 677) and R-125 (GWP 3170), with an azeotropic composition at 91 mol% R-32 (GWP 677).
The research on technologies based on environmentally benign materials that efficiently capture, separate, and recycle F-gases is vital to develop sustainable processes to reduce the environmental impact of refrigerants based on F-gases. The implementation of innovative approaches based on separation processes is vital to the functioning of a sustainable market under circular economy principles. However, few projects have been dedicated to exploring the separation of HFCs from HFC blends. Recently, under the scope of the KET4F-Gas project
[47], two technologies, currently in the patent process, have been developed and prototypes have been constructed for the efficient recovery of value-added HFCs, with purity up to 99%, from high-GWP refrigerant blends contained in EoL equipment, for reutilization purposes in novel environmentally friendly low-GWP blends. These technologies are easy to apply in a waste management facility due to the small space required, and due to their modularity and scalability. Moreover, these systems require low maintenance and have a long lifetime
[48][49]. One of the technologies is based on adsorption processes, in which one of the components of the mixture is preferentially adsorbed into a porous material while the remaining leaves the adsorption column, including the regeneration of the adsorption column to recover the adsorbed gas
[50]. The other technology is based on the separation by a membrane, in which one compound of the mixture is preferentially permeated through highly selective thin films due to differences in the size of the gas molecules and to gas–membrane interactions
[51].
This example shows that innovation in the management of F-gases recovered from EoL equipment is possible and is essential to the goals of reducing the contribution of these gases to climate change. Actions, such as investment in the search for new separation technologies, need to be performed in the management of F-gases since most are recovered and destroyed at the end-of-life of the equipment, representing a loss of value-added compounds.