
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonino Romano | + 3089 word(s) | 3089 | 2020-06-04 08:26:14 | | | |
| 2 | Rita Xu | -826 word(s) | 2263 | 2020-06-10 11:12:38 | | | | |
| 3 | Rita Xu | -26 word(s) | 2237 | 2020-10-27 09:49:51 | | |
Video Upload Options
Iron deficiency (ID) is the most frequent nutritional deficiency in the whole population worldwide, and the second most common cause of anemia in the elderly. Anemia in aged subjects impacts health and quality of life, and it is associated with several negative outcomes, such as longer time of hospitalization and a higher risk of disability. Furthermore, it is an independent risk factor of increased morbidity and mortality. Even though iron deficiency anemia is a common disorder in older adults, it should be not considered as a normal ageing consequence, but a sign of underlying dysfunction. Relating to the pathophysiology in Iron Deficiency Anemia (IDA), hepcidin has a key role in iron homeostasis. It downregulates the iron exporter ferroportin, inhibiting both iron absorption and release. Furthermore, IDA at old age is frequently dependent on blood loss, especially caused by gastrointestinal lesions. Thus, a diagnostic algorithm for IDA should include invasive investigation such as endoscopic procedures. The treatment choice is influenced by the severity of anemia, underlying conditions, comorbidities, and the clinical state of the patient. Correction of anemia and iron supplementation should be associated with the treatment of the causal disease.
1. Pathophysiology
1.1. Etiology
It is still unclear how physiological changes due to ageing may affect iron metabolism because available data are few and contrasting. At the moment, there is no demonstration that the increased incidence of IDA in the elderly is related to ageing physiological evolution [1]. The iron regulatory hormone hepcidin may play a role in the development of anemia in the elderly. Hepcidin, stimulated by inflammation cytokines, prevents iron release from macrophages and hepatocytes and inhibits the iron transport to plasma from the enterocytes of the proximal duodenum, increasing iron retention in the reticuloendothelial system [1][2][3]. Furthermore, there is an impaired erythropoietin production in response to anemia [4].
In 2018 the German Geriatric Society released two position papers on iron deficiency anemia in elderly, to place it in the wider context of geriatric syndromes. In the first paper, Röhrig et al. define IDA as a very common disorder in aged people, but one that was not caused by age-related physiological changes [5] In the second paper, IDA is described as a geriatric syndrome, and thus similar to sarcopenia and frailty [6]. The complete absence of iron stores can be defined “absolute IDA”, whereas the terms “functional” or “relative” may indicate normal or higher iron stores with iron-restricted erythropoiesis, respectively. Even though IDA is a common disorder in older adults, it should not be considered as a normal ageing consequence, because it is usually a sign of an underlying disease. In the elderly, anemia is usually multifactorial (Figure 1).
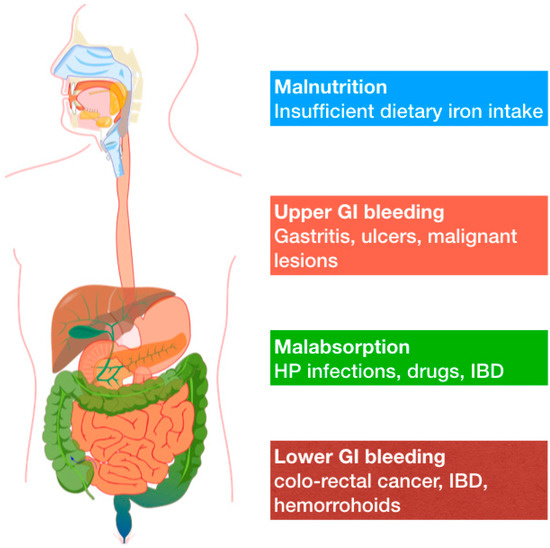
Figure 1. Common causes of IDA in adults include a broader range of potential mechanisms that are here synthetically depicted.
Definitive diagnosis of the cause of anemia in the elderly can be challenging, even if the main cause of anemia is identified in about 80% of cases. The prevalent cause of anemia in the geriatric population is ID. Nutrient deficiencies are implicated in one-third of anemia cases (and more than 50% of these are related to iron deficiency). Chronic diseases and chronic inflammations, are involved in about another one-third of anemia cases. Ageing is associated with a chronic low-grade inflammation and this has been named “Inflammaging” [7]. Inflammation in the elderly is associated with changes in body composition, immunosenescence, metabolic status and it is predictive of increased morbidity and mortality. The remaining 20–30% of anemia is defined as “unexplained anemia of the elderly” (UAE) or “idiopathic cytopenia of unknown significance” (ICUS), and is characterized by a hypoproliferative normocytic anemia in patients with no chronic diseases, inflammatory disorders, or nutritional deficiencies [1][8][9][10][11][12]. Gowanlok et al., studying a group of 570 patients older than 60 years, observed that erythropoietin levels were inappropriately low in anemia of unknown etiology, hypothesizing that decreased erythropoietin production may play a key role in the pathogenesis of this category of anemia [13][14]. The diagnosis of UEA may be considered after excluding other possible causes (including hematological disorders) [4]. In fact, the incidence of myelodysplastic syndrome in older people is high, rising to 36.4 cases/100.000 population per year in those over 80 years, and increasing further with ageing. The median age at diagnosis of myelodysplastic syndrome is 76 years. Long-term treatments with non-steroidal anti-inflammatory drugs, antithrombotic drugs, anticoagulants, proton pump inhibitors, corticosteroids, iron-free erythropoietin, may also be relevant causes of ID in the elderly [2][4][11][15].
Finally, low dietary iron intake could be a possible cause of ID. There are two types of dietary iron: heme form and non-heme form. Heme form is most prevalent in meat and meat products, oily fish, cereal products, eggs, pulses and dark green vegetable. Iron absorption is facilitated by vitamin C intake and prevented by coffee consumption. The evaluation of the effective amount of dietary iron intake is challenging in the elderly, as impaired adsorption and malnutrition are common [3][16].
1.2. Molecular Mechanisms
Systemic and cellular iron levels are tightly regulated. Iron is transported around the body by plasma transferrin and delivered to cells through an endocytotic process [17]. Within cells, iron is stored in ferritin, with hepatocytes and macrophages being particularly important sites of iron storage. Quantitatively, most iron is used for hematopoiesis, but all body cells have a requirement for iron. When demand is higher, iron is released from cells through the only known iron exporter, ferroportin [18]. Ferroportin is in turn controlled by hepcidin, a 25 amino acid peptide hormone secreted by the liver, which negatively regulates ferroportin by facilitating its internalization and degradation. As a result, iron accumulates within cells [19]. Thus, hepcidin plays a key role in iron homeostasis. As a playmaker of systemic iron balance, hepcidin transcription in the liver is fine controlled by multiple signals, especially erythropoietic drive, iron deficiency and inflammation [19]. In hepcidin-knock-out mice, iron accumulates in the liver, pancreas and heart, while plasma iron levels are reduced [20][21]. Overexpression of hepcidin in transgenic mice results in IDA with severe microcytemia [21]. Such observations have also been reported in humans. Patients with hepatic adenomas and anemia scarcely respond to iron replacement therapy, and have high levels of hepcidin expression and lower serum transferrin saturation [22]. When tissue and circulating iron levels are elevated, hepcidin transcription is upregulated to limit further iron entry, while hepcidin expression is downregulated with an increased erythropoietic drive. As noted above, in the elderly, low-grade inflammation is common. Acute inflammation contributes to the severity of anemia in the context of hospitalization. Multiple mechanisms are involved in the pathogenesis of anemia. Inflammatory cytokines such as TNF-α, IL-6, IL-β, IL-γ both slow erythropoiesis and increase hepcidin levels. IL-6 induces hepcidin transcription in response to multiple infections including streptococcus pneumonia and influenza A. IL-6-knockout mice demonstrated impaired or absent hepcidin induction in response to an inflammatory stimulus [23]. Such observations have important implications in the management of anemia in the elderly [24]. In these patients, a high level of IL-6 is expected to stimulate hepcidin expression which, in turn, induces iron retention in macrophages and reduces erythropoiesis [25]. Similar findings were demonstrated in dialyzed patients with end-stage renal disease [26].
2. Diagnosis
Accurate collection of a patient’s history (comorbidities, pharmacological therapy, symptoms), clinical evaluation, and laboratory tests are needed to perform a definitive and differential diagnosis of anemia. Laboratory tests should include serum Hb, complete blood count, mean cell volume (MCV), mean corpuscular hemoglobin (MCH), reticulocyte count, plasma ferritin, serum transferrin saturation, serum iron, serum folate and vitamin B12, serum copper, circulating inflammatory markers (such as C-reactive protein, and fibrinogen), and organ function markers as serum creatinine/eGFR, serum aminotransferases, serum electrophoresis, thyreotropin (TSH), lactate dehydrogenase (LDH), serum erythropoietin (EPO) [4][11]. Mild ID is characterized by a reduction of the transferrin saturation, with a normal Hb value. Severe ID is characterized by microcytic, hypochromic anemia [2][3].
Bone marrow puncture is the gold standard procedure to diagnose IDA [27]. This technique is crucial to diagnose eventual myelodysplasia, but it is not routinely used in aged and fragile patients because of its invasiveness. IDA is associated with microcytosis (MCV <80 fL). However, in the elderly, a vitamin B12 and folate deficiency may often occur, leading to an increase in MCV with resulting normocytic anemia that can complicate the interpretation of laboratory data. As a consequence, MCV assessment is not useful to rule out an IDA in the elderly, especially if patients present with comorbidities. Serum iron and transferrin saturation are the main indicators of adequate iron supply. The presence of low serum iron concentration (<33 g/dL) and transferrin saturation <15%, increased iron-binding capacity (>400 g/dL), and low serum ferritin value (≥50 μg/mL) is diagnostic for ID. However, the sensitivity of these parameters is lower in aged than in younger adult patients. In addition to being a marker of iron stores, plasma ferritin is an acute-phase marker, and may be increased in cases of systemic inflammation, infections, and chronic disorders. Thus, when serum markers of inflammation are higher, a serum ferritin lower than 100 μg/L can be strongly suggestive of ID. A serum ferritin lower than 12 μg/L indicates a lack of iron stores. Serum ferritin should be assessed in conjunction with other inflammatory markers (such as C-reactive protein, erythrocyte sedimentation rate, and fibrinogen), since its concentration can be elevated by inflammation and chronic disorders. An alternative marker of iron deficiency is the level of soluble transferrin receptor (sTfR) in serum. sTfR levels reflect erythropoietic activity and the demand of the bone marrow for iron. It increases in conditions of low iron concentration. Its use can be helpful to differentiate IDA from anemia caused by chronic diseases or inflammatory diseases, or by an interaction of both conditions. But its employment is limited because of the lack of standardized reagents in clinical laboratories, and the absence of a specific cut-off [2][8][11][28][29]. A situation of low serum iron associated with low transferrin saturation and high serum ferritin, with low total iron-binding capacity, is very suggestive of anemia of chronic diseases [30].
Reticulocyte count and reticulocyte index can help to differentiate between hypoproliferative anemia, and the presence of blood loss, hemolysis, hemoglobinopathies, or disorders in red cell structure and enzymes. In fact, the first condition is characterized by a reduction in reticulocytes, while the following is present with an increased red blood cells generation, because of bone marrow compensation. A reticulocyte index ≥2 is related to blood loss or hemolysis; if lower than 2, this indicates bone marrow failure, iron deficiency, or poor erythropoietin production. In elderly anemia, reticulocytes are usually low [10][30]. Mean reticulocyte hemoglobin content (CHr) and the reticulocyte hemoglobin equivalent (Ret-He) are indexes of the hemoglobin content in the reticulocytes. Low CHr or Ret-He values might be an early marker of iron-deficient erythropoiesis. They correlate with a lower iron supply for the red blood cell compartment in the bone marrow. Even though there are limited data about geriatric patients measurement of the Ret-He level is no more specific than other red cell indices (such as MCH and MCHC) in discriminating iron deficiency anemia from the anemia of chronic disease in the elderly [11].
The evaluation of serum hepcidin levels may prove a useful tool in distinguish IDA from the anemia associated with inflammatory and chronic diseases, but it requires further validation and is not yet routinely used in clinical practice.
In assessing IDA in the elderly, it is necessary to determine whether there are any underlying disorders that may cause iron deficiency. Chronic upper (40–60%) and lower (15–30%) gastrointestinal blood loss (as peptic ulcers, malignancies, esophagitis, varices in portal hypertension, gastritis, inflammatory bowel diseases, polyps, hemorrhoidal bleeding) are the most frequent conditions in older adults that could lead to IDA. Other causes are arteriovenous malformations (intestinal angiodysplasia is the most frequent one), extra-intestinal blood losses such as hematuria or gynecological bleeding, and other occult malignancies. Even though it is less frequent in industrialized countries, IDA could be related to nutritional deficiency, caused by malnutrition and insufficient iron intake, or by malabsorption disorders like celiac disease (CD) or achlorhydria. With particular regards to celiac disease, intestinal symptoms are less frequent in the elderly than in younger people. Moreover, micronutrient deficiencies may be prominent and sometimes the only presentation of the disease is in the elderly. CD is a cause of anemia, because of the malabsorption of iron, folic acid, and vitamin B12. Nevertheless, the impairment in iron absorption is the main driver of IDA in celiac elderly and often anemia is the only manifestation of the CD. Therefore, such observations reinforce the need for screening for CD in older patients with IDA [31][32].
Other conditions to consider in the differential diagnosis of IDA in the elderly are alcohol abuse, malnutrition, and chronic diseases such as chronic renal failure and inflammatory diseases [33][34][35][36][37].
Since blood loss commonly accounts for IDA in the elderly, the diagnostic algorithm should include endoscopic procedures, as esophagogastroduodenoscopy, colonoscopy, and possibly video capsule endoscopy. Indeed, 20% of elderly patients affected by IDA present with a negative upper and lower intestine endoscopy, and two-thirds of these patients suffer from a small bowel lesion. Thus, these investigations are recommended even with a negative occult fecal blood test result, because bleeding can be discontinuous. Old age is not an absolute contraindication to endoscopic procedures, but every patient needs to be evaluated, taking into consideration clinical conditions such as frailty and comorbidities. In selected cases, CT colonography (virtual colonoscopy) may be considered, even if its accuracy is lower than endoscopic investigations [4][11][33][36][38]. According to Andrès et al., iron deficiency in very old frail patients and in patients with life-threatening diseases should be treated with iron supplementation, avoiding preliminary invasive investigations [36].
References
- David P. Steensma; Ayalew Tefferi; Anemia in the Elderly: How Should We Define It, When Does It Matter, and What Can Be Done?. Mayo Clinic Proceedings 2007, 82, 958-966, 10.4065/82.8.958.
- Fabiana Busti; Natascia Campostrini; Nicola Martinelli; Domenico Girelli; Iron deficiency in the elderly population, revisited in the hepcidin era. Frontiers in Pharmacology 2014, 5, 83, 10.3389/fphar.2014.00083.
- Susan Fairweather-Tait; Anna Wawer; Rachel Gillings; Amy Jennings; Phyo K. Myint; Iron status in the elderly. Mechanisms of Ageing and Development 2014, 136, 22-28, 10.1016/j.mad.2013.11.005.
- Reinhard Stauder; Peter Valent; Igor Theurl; Anemia at older age: etiologies, clinical implications, and management. Blood 2018, 131, 505-514, 10.1182/blood-2017-07-746446.
- Gabriele Röhrig; Ines Gütgemann; Gerald Kolb; Andreas Leischker; Anemia in the aged is not ageing related: position paper on anemia in the aged by the “working group anemia” of the German Geriatric Society (DGG). European Geriatric Medicine 2018, 9, 395-397, 10.1007/s41999-018-0048-0.
- Rohrig, G.; Gutgemann, I.; Leischker, A.; Kolb, G; Anemia in the aged—A geriatric syndrome?: Second position paper on anemia in the aged by the working group anemia of the German Geriatric Society. Z. Gerontol. Geriatr. 2018, 51, 921–923.
- Claudio Franceschi; Paolo Garagnani; Paolo Parini; Cristina Giuliani; Aurelia Santoro; Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nature Reviews Endocrinology 2018, 14, 576-590, 10.1038/s41574-018-0059-4.
- Lawrence Tim Goodnough; Stanley L. Schrier; Evaluation and management of anemia in the elderly.. American Journal of Hematology 2014, 89, 88-96, 10.1002/ajh.23598.
- Racha Halawi; Hassan Moukhadder; Ali T. Taher; Anemia in the elderly: a consequence of aging?. Expert Review of Hematology 2017, 10, 327-335, 10.1080/17474086.2017.1285695.
- Van Puyvelde, K.; Cytryn, E.; Mets, T.; Beyer, I; Anaemia in the elderly. BMJ 2009, 64, 292–302.
- Etienne Joosten; Iron deficiency anemia in older adults: A review. Geriatrics & Gerontology International 2017, 18, 373-379, 10.1111/ggi.13194.
- Francesco Bellanti; Giuseppina Iannelli; Maria Blonda; Rosanna Tamborra; Rosanna Villani; Adele Romano; Silvio Calcagnini; Gianluigi Mazzoccoli; Manlio Vinciguerra; Silvana Gaetani; et al.Anna Maria GiudettiGianluigi VendemialeTommaso CassanoGaetano Serviddio Alterations of Clock Gene RNA Expression in Brain Regions of a Triple Transgenic Model of Alzheimer’s Disease. Journal of Alzheimer's Disease 2017, 59, 615-631, 10.3233/jad-160942.
- Zachary Gowanlock; Swetha Sriram; Alison Martin; Anargyros Xenocostas; Alejandro Lazo-Langner; Erythropoietin Levels in Elderly Patients with Anemia of Unknown Etiology. PLOS ONE 2016, 11, e0157279, 10.1371/journal.pone.0157279.
- Francesco Bellanti; Antonino D. Romano; Aurelio Lo Buglio; Valeria Castriotta; Giuseppe Guglielmi; Antonio Greco; Gaetano Serviddio; Gianluigi Vendemiale; Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity.. Maturitas 2017, 109, 6-12, 10.1016/j.maturitas.2017.12.002.
- Stauder, R.; Valent, P.; Theurl, I; nemia at older age: Etiologies, clinical implications, and management. Blood 2018, 131, 505–514.
- Darius Lane; Patric Jansson; Des R. Richardson; Bonnie and Clyde: Vitamin C and iron are partners in crime in iron deficiency anaemia and its potential role in the elderly. Aging 2016, 8, 1150-1152, 10.18632/aging.100966.
- Yujie Cui; Qingyu Wu; Yiqing Zhou; Iron-refractory iron deficiency anemia: new molecular mechanisms. Kidney International 2009, 76, 1137-1141, 10.1038/ki.2009.357.
- Andrews, N.C.; Schmidt, P.J; Iron homeostasis. Annu. Rev. Physiol. 2007, 69, 69–85.
- Tomas Ganz; Hepcidin and Its Role in Regulating Systemic Iron Metabolism. Hematology 2006, 2006, 29-35, 10.1182/asheducation-2006.1.29.
- Gaël Nicolas; Myriam Bennoun; Isabelle Devaux; Carole Beaumont; Bernard Grandchamp; Axel Kahn; Sophie Vaulont; Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proceedings of the National Academy of Sciences 2001, 98, 8780-8785, 10.1073/pnas.151179498.
- Gaël Nicolas; Myriam Bennoun; Arlette Porteu; Sandrine Mativet; Carole Beaumont; Bernard Grandchamp; Mario Sirito; Michèle Sawadogo; Axel Kahn; Sophie Vaulont; et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proceedings of the National Academy of Sciences 2002, 99, 4596-4601, 10.1073/pnas.072632499.
- David Weinstein; Cindy N. Roy; Mark D. Fleming; Massimo F. Loda; Joseph I Wolfsdorf; Nancy Andrews; Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood 2002, 100, 3776-3781, 10.1182/blood-2002-04-1260.
- Richard Rodriguez; Chun-Ling Jung; Victoria Gabayan; Jane C. Deng; Tomas Ganz; Elizabeta Nemeth; Yonca Bulut; Hepcidin Induction by Pathogens and Pathogen-Derived Molecules Is Strongly Dependent on Interleukin-6. Infection and Immunity 2014, 82, 745-752, 10.1128/iai.00983-13.
- Antonino Davide Romano; Eulalia Greco; Gianluigi Vendemiale; Gaetano Serviddio; Bioenergetics and mitochondrial dysfunction in aging: recent insights for a therapeutical approach.. Current Pharmaceutical Design 2014, 20, 2978-2992, 10.2174/13816128113196660700.
- H Ludwig; E Fritz; C Leitgeb; M Pecherstorfer; H Samonigg; J Schuster; Prediction of response to erythropoietin treatment in chronic anemia of cancer. Blood 1994, 84, 1056–1063.
- Sai Ram Keithi-Reddy; Francesco Addabbo; Tejas V. Patel; Bharati V. Mittal; Michael S. Goligorsky; Ajay K. Singh; Association of anemia and erythropoiesis stimulating agents with inflammatory biomarkers in chronic kidney disease. Kidney International 2008, 74, 782-790, 10.1038/ki.2008.245.
- Besarab, A.; Hemmerich, S. Iron-Deficiency Anemia. In Management of Anemia; Springer: New York, NY, USA, 2017; pp. 11–29.
- Inês Rangel; Alexandra Gonçalves; Carla De Sousa; Sergio Leite; María Díez Campelo; Elisabete Martins; Sandra Amorim; Brenda Moura; José Silva Cardoso; Maria Júlia Maciel; et al. Iron Deficiency Status Irrespective of Anemia: A Predictor of Unfavorable Outcome in Chronic Heart Failure Patients. Cardiology 2014, 128, 320-326, 10.1159/000358377.
- Abitbol, V.; Borderie, D.; Polin, V.; Maksimovic, F.; Sarfati, G.; Esch, A.; Tabouret, T.; Dhooge, M.; Dreanic, J.; Perkins, G.; et al.et al Su1293 Diagnosis of Iron Deficiency in Inflammatory Bowel Diseases by Transferrin Receptor-Ferritin Index. Index. Medicine 2015, 94, e1011.
- Lodovico Balducci; W.B. Ershler; S. Krantz; Anemia in the elderly—Clinical findings and impact on health. Critical Reviews in Oncology/Hematology 2006, 58, 156-165, 10.1016/j.critrevonc.2005.09.003.
- Rashtak, S.; Murray, J.A; Celiac Disease in the Elderly. Gastroenterol. Clin. N. Am. 2009, 38, 433–446.
- Martín- Masot; Teresa Nestares; Diaz- Castro; López- Aliaga; María J. M. Alférez; Moreno- Fernandez; José Maldonado; Rafael Martín-Masot; Javier Díaz-Castro; Inmaculada López-Aliaga; et al.Jorge Moreno-Fernandez Multifactorial Etiology of Anemia in Celiac Disease and Effect of Gluten-Free Diet: A Comprehensive Review. Nutrients 2019, 11, 2557, 10.3390/nu11112557.
- Adnan Muhammad; Gitanjali Vidyarthi; Patrick Brady; Role of small bowel capsule endoscopy in the diagnosis and management of iron deficiency anemia in elderly: A comprehensive review of the current literature. World Journal of Gastroenterology 2014, 20, 8416-8423, 10.3748/wjg.v20.i26.8416.
- Therese McNamee; Trish Hyland; Janas Harrington; Sharon Cadogan; Bahman Honari; Kanthi Perera; Anthony P. Fitzgerald; Ivan J. Perry; Mary R. Cahill; Haematinic Deficiency and Macrocytosis in Middle-Aged and Older Adults. PLOS ONE 2013, 8, e77743, 10.1371/journal.pone.0077743.
- Jui-Line Wang; Ning-Sing Shaw; Iron status of the Taiwanese elderly: the prevalence of iron deficiency and elevated iron stores.. Asia Pacific Journal of Clinical Nutrition 2005, 14, 278–284.
- Olivier Keller And Emmanuel Andrès; Khalid Serraj; Laure Federici; Thomas Vogel; Georges Kaltenbach; Anemia in elderly patients: New insight into an old disorder. Geriatrics & Gerontology International 2013, 13, 519-527, 10.1111/ggi.12017.
- Ingo Beyer; N. Compté; A. Busuioc; S. Cappelle; C. Lanoy; E. Cytryn; Anemia and transfusions in geriatric patients: a time for evaluation. Hematology 2010, 15, 116-121, 10.1179/102453310x12583347010052.
- Romano, A.D.; Serviddio, G.; de Matthaeis, A.; Bellanti, F.; Vendemiale, G; Oxidative Stress and Aging. J. Nephrol. 2010, 23, S29–S36.




