
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mingfei Pan | + 4497 word(s) | 4497 | 2020-06-01 10:38:03 | | | |
| 2 | Catherine Yang | Meta information modification | 4497 | 2020-06-04 04:32:26 | | | | |
| 3 | Catherine Yang | -84 word(s) | 4413 | 2020-10-28 03:36:03 | | |
Video Upload Options
Carbon quantum dots (CQDs) with stable physicochemical properties and excellent optical performances are a kind of emerging and fascinating carbon nanomaterial with size less than 10 nm. The significant advantages of low cytotoxicity and cost make CQDs an ideal raw material for constructing effective sensing devices. The CQDs can be functionalized and combined with other kinds of materials to form the nanostructured composites with unique properties, having a very broad application prospect for related research of many fields. Green-synthesized CQDs have been applied in functional components analysis and monitoring trace harmful substances in food and made remarkable research progress. This entry reviews the relevant sensing applications of CQDs in food components analysis and food safety inspection of recent five years, which can provide significant references for further study of fluorescent and biomimetic sensing of CQDs in this field.
With the continuous deepening of researches on related techniques, carbon quantum dots (CQDs) with different performances have been synthesized and functionalized and then successfully applied in many research fields and especially in the sensing analysis, which have made remarkable research progress [1][2]. In terms of food analysis, CQDs with excellent performances have been used as sensing probes in food components (such as phenolic compounds, saccharides, vitamins, proteins, amino acids, etc.) or harmful substances (such as pesticides and veterinary drugs residues, illegal additives, heavy metals, mycotoxins, etc.) [3][4][5]. This type of studies not only deepens the basic theoretical research of CQDs but also expands the related applications of CQDs. It also provides new strategies for the analysis of components in food and the rapid detection of trace harmful substances.
1. Functional Components in Foods
In the field of food analysis, CQDs acts as a sensing probe to detect and analyze functional components of food such as protein, vitamins, phenolic compounds and so on, which not only has excellent optical properties but also has significant advantages of pro-environment, low price, convenience and speediness. Foods can provide enough energy and nutrients for human body, such as protein, fat, carbohydrates, vitamins and minerals, which are essential for normal life activities. As a result, the detection of nutrients in foods is very necessary.
The content of ovalbumin (OVA) is deemed as a reference for evaluating the quality of protein [6]. Fu et al. synthesized a novel CQDs co-doped with N, O, P (NOP-CQDs) through one-step hydrothermal method and applied for quantitative detection of OVA in the egg products [7] (Figure 1a). In the fluorescent resonance energy transfer (FRET) system composed of NOP-CQDs, graphene oxide and anti-OVA, the “on-off” sensing probe has achieved the selective recognition and capture of OVA based on the specific interaction of antigen-antibody, which achieved a limit of detection (LOD) of 153 µg L−1. Purbia et al. developed a highly luminescent CQDs (Size: 1–6 nm) with green and blue fluorescent for detecting vitamin B1 in commercial vitamin capsule (LOD: 280 nmol L−1) [8]. The detection principle is that the gradual addition of vitamin B1 can restore the fluorescent of CQDs quenched by Cu2+, thereby achieving the rapid detection of targeted component. Certain small molecular substances present in plant-derived foods have specific antibacterial, anti-inflammatory or anti-oxidant properties, which gives the foods special medicinal properties. These small molecules are defined as “functional components” and this kind of foods is called “medicine-homologous foods” [9][10]. Qualitative or quantitative analysis of the functional components in medicinal-homologous foods is usually the main method to evaluate their quality. Fluorescent CQDs provides an effective, convenient and accurate strategy for the analysis and detection of such efficacy components. Chlorogenic acid has remarkable antibacterial and antiviral pharmacological effects, the amount of which is the main basis for evaluating the quality of honeysuckle (a medicinal-homologous food). Yang et al. synthesized the water-soluble CQDs with size of 2.1 nm and quantum yield (QY) of 16.5% via hydrothermal treatment of malic acid and urea and applied for the fluorescent sensing detection of chlorogenic acid in honeysuckle [11] (Figure 1b). Due to the mechanism of internal filter effect (IFE) [12], the fluorescent of CQDs is effectively quenched as the concentration of chlorogenic acid increases in the linear range of 0.15–60 µmol L−1, with a lower LOD of 45 nmol L−1. By comparison with the high performance liquid chromatography (HPLC) and HPLC-mass spectrometry (HPLC-MS/MS) for chlorogenic acid detection [13][14], the developed CQDs-based method not only has good improvement on the sensitivity but also significantly improves the detection speed, which is suitable for rapid screening of large quantities of honeysuckle samples. Curcumin (Cur) is an acidic polyphenolic substance extracted from the roots of ginger plants and its main chain is unsaturated aliphatic and aromatic groups. As a yellow pigment, it is often used as a meat coloring agent and acid-base indicator and has anti-inflammatory, antioxidant and other pharmacological effects [15][16]. Liu et al. have rapidly synthesized one N and P dual-doped CQDs (NP-CQDs) using glucose as carbon source, 1,2-ethylenediamine as N-dopant and concentrated phosphoric acid as P-dopant, which was further utilized as a label-free sensor for Cur determination [17] (Figure 1c), achieving a linear range of 0.5–20 µmol L−1 and a LOD of 58 nmol L−1. In practical samples (drinking water and foods), satisfactory relative standard deviations (RSD) and recoveries were 0.08–5.39% and 95.2–105.2%, respectively. Additionally, this NP-CQDs can be used as effective fluorescent agent for cellular imaging without noticeable cytotoxicity.
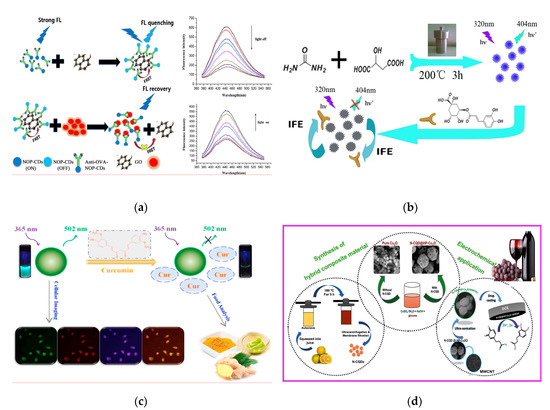
Figure 1. (a) Schematic of the quantitative detection for ovalbumin (OVA) based on fluorescent resonance energy transfer (FRET) of N, O, P co-doped CQDs (NOP-CQDs). Reproduced with permission from [7]. Copyright Sensors and Actuators B: Chemical, 2018; (b) Schematic of the detection of chlorogenic acid in honeysuckle using CQDs. Reproduced with permission from [11]. Copyright Spectrochimica Acta Part A-molecular and Biomolecular Spectroscopy, 2018; (c) Schematic of the quenching mechanism of Curcumin to NP-CQDs and the applications of cellular imaging. Reproduced with permission from [17]. Copyright Talanta, 2018; (d) Schematic of the synthesis pathway of the composite (CQDs@HP-Cu2O/MWCNT) composed of CQDs, hexagonal porous Cu2O (HP-Cu2O) and multi-walled carbon nanotubes (MWCNT) and caffeic acid detection process. Reproduced with permission from [18]. Copyright Composites Part B-Engineering, 2019.
Based on the luminescent characteristic of CQDs and the selective adsorption of molecularly imprinted polymers (MIPs), CQDs-embedded MIPs has provided new methods for the fluorescent analysis of trace substances in complex food matrices. Using the metal organic frameworks (MOFs) as the core of surface molecular imprinting, Xu et al. designed a novel nanocomposite of CQDs@MOF@MIP and further developed a fluorescent sensor for the detection of quercetin (QCT) in extract capsule of Ginkgo biloba. The fluorescent sensor showed remarkable sensitivity and selectivity to QCT in the wide concentration range of 0–50.0 µmol L−1 with a LOD of 2.9 nmol L−1 (S/N = 3) [19]. This CQDs@MOF@MIP sensing model has high specific surface area and ample cavities and further possesses the ability of signal amplification and conversion, which can transform chemical signal into the detectable fluorescent signal by binding with target molecules, potentially becoming an innovative technology. Figure 1d has shown the synthesis process of the composite composed of the N-CQDs decorated hexagonal porous Cu2O and multi-walled carbon nanotubes (MWCNT) and the application of N-CQDs@HP-Cu2O/MWCNT for the detection of caffeic acid (CA) in red wine [18]. The fabricated fluorescent sensing device was demonstrated to possess high sensitivity, good repeatability and stability to CA. The doped CQDs and conductive MWCNT make the composite have higher specific surface area and porosity and further improve the electrocatalytic activity of Cu2O-based materials [20][21]. This study provides a strong guidance for the fabrication of various porous nanocomposites. Rutin is a flavonol glycoside widely present in plants and can be used as an edible antioxidant and nutrition enhancer. Sinduja et al. synthesized CQDs (Size: 7 nm) using the non-essential amino acid asparagine as a precursor and further exploited it for the determination of rutin by spectrofluorimetry based on the decrease in emission intensity at 441 nm [22]. Good linear relationship (R2 = 0.997) was obtained in the range of 0.5–15 µmol L−1 with a LOD of 0.1 µmol L−1. In this study, the intrinsic fluorescent characteristic of CQDs and the selective π-π interaction between rutin and the CQDs aromatic rings enhance the detection accuracy and reliability to the target.
2. Poisonous and Harmful Substances in Foods
2.1. Pesticide and Veterinary Drug Residues
The residues of pesticides and veterinary drugs in foods, including the drug prototypes and their metabolites that accumulate in foods, are potentially harmful to human health [23]. The effective analysis to these residues is one important topic in the research of food safety. The emerging CQDs material can offer more accurate, efficient and cost-effective fluorescent sensing, which has made great progress in the detection of pesticides and veterinary drugs residues in food in recent years [24][25].
The MIPs with specific recognition ability are commonly used for the purification of complex matrices and the enrichment and separation of trace target molecules of pesticides and veterinary drugs in foods. The emerging CQDs-embedded MIPs have provided a very meaningful strategy of the detection of small molecule harmful substances in foods. Zhang and the co-workers have successfully designed a room-temperature ionic-liquid (RTIL)-sensitized CQDs sensing probe (RTIL-SCQDs-MIPs) and applied for sensitive detection of insecticide lambda-cyhalothrin (LC) in vegetable and tea samples, with a LOD of 0.5 µg kg−1 [26]. It has demonstrated that the introduced S-doped CQDs and RTIL in the sensing probe can enhance the performances of recognition and sensing. Based on the mechanism of host-guest interactions [27], the outer MIPs-based material plays an important role in the identification of target LC in tested samples, which also provides a very promising prospect for the specific identification of trace targets in complex food matrices. This type of composites has combined the advantages of a single material with different functions to form a synergistic enhancement effect and provides a very interesting strategy in the detection of small molecules.
Wu et al. have developed a vinyl phosphate (VPA)-functionalized, magnetic MIP (MMIP) microspheres for the enrichment of organophosphorus pesticide residues and further combined with CQDs for fluorescent detection [28] (Figure 2a). Dual functional monomers and mesoporous SiO2-modified Fe3O4 magnetic particles (Fe3O4@mSiO2) have prominent advantages in the formation of recognition cavities in MIPs, which further improve the sensing properties of the hybrid composites. This MMIP-CQDs@VPA fluorescent sensor was further applied for the detection of triazophos in cucumbers with a lower LOD of 0.0015 mmol L−1, which was proved to possess high accuracy, good sensitivity and repeatability. Fu et al. have constructed one fluorescent sensor based the composite of CQDs and Fe3+ which has good response to ampicillin in mineral water, milk and pork samples with a LOD of 0.7 µmol L−1 [29]. This merit of the research benefits by the binding sites provided by the surface functional groups of CQDs for the metal ion Fe3+ and this sensor offers more feasible and promising method in the simultaneous detection of multiple targets. Compared to the nano-optical sensor based on the core-shell polypyrrole-CdTe QDs-MIP [30], the CQDs/Fe3+-based sensor did not need the complicated synthesis process and long reaction time.
Importantly, the use of CQDs to take place of heavy metals-based QDs has reduced the potential toxicity in practical applications. Li et al. fabricated the single-hole hollow MIP-CQDs fluorescent sensor (HMIP@CQDs) using sol-gel method for sensitive and rapid detection of tetracycline (TC) in honey samples [31] (Figure 2b). In this study, HMIP was employed as a recognition element for the targeted recognition and fluorescent detection of TC (LOD: 3.1 µg L−1, Recovery: 93–105%). The HMIP@CQDs showed better fluorescent performance than the traditional solid core MIP@CQDs [32]. Mahmoud and the co-workers have designed an electrochemical sensor based on the composite MIP-AuNPs/NS@GQDs, which has good sensitivity for detecting antiviral drug sofosbuvir [33]. Due to the successful electro-polymerization of NS@GQDs and AuNPs on pencil graphite electrodes, good conductivity and electrocatalytic activity were obtained in the electrochemical sensing process.
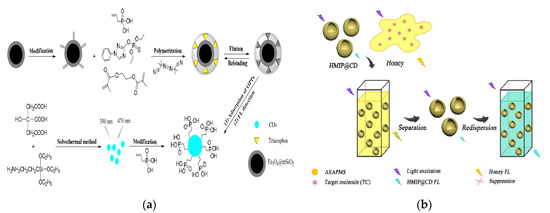
Figure 2. (a) Schematic of the preparation of core-shell magnetic molecularly-imprinted microspheres (MMIPs) of vinyl phosphate (VPA)-functionalized CQDs (MMIPs-CQDs@VPA) and the detection process for triazophos. Reproduced with permission from [28]. Copyright Polymers, 2019; (b) Schematic of fluorescent detection process of tetracycline (TC) in honey. Reproduced with permission from [31]. Copyright Talanta, 2018.
Noble metal NPs, such as AuNPs and AgNPs, have a large specific surface area and low energy transfer resistance and are an ideal carrier of electrochemical sensing [34][35][36]. The composite materials with CQDs provide a better sensing interface for electrochemical and fluorescent sensing, which can achieve higher sensitivity and accuracy in the detection of harmful substances in foods. A novel fluorescent aptasensor composed of AuNPs and CQDs was fabricated for sensitively and selectively detecting acetamiprid pesticides in tomato, cucumber and cabbage sample [37] (Figure 3a). One aptamer S-18 (recognition element) was introduced to combine with AuNPs to effectively quench the fluorescent of CQDs to achieve the detection of target (LOD: 1.08 µg L−1), which provides a new idea for the construction of specific and functionalized fluorescent sensors. An electrochemical sensing platform was constructed using the composite of Ag/Ag2O@NS-CQDs for the detection of catechol, achieving a low LOD of 13 nmol L−1 [38]. It has demonstrated that the Ag/Ag2O@NS-CQDs composite has better conductivity, specific surface area and electrocatalytic ability than that of NS-CQDs and its participation greatly increases the electrochemical activity on the sensing interface, which has a remarkable guiding significance for the construction of various electrochemical sensors with excellent performance.
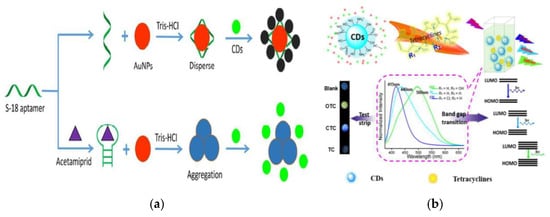
Figure 3. (a) Schematic of the fluorescent aptasensor for acetamiprid detection based on AuNPs and CQDs. Reproduced with permission from [37]. Copyright Analyst, 2018; (b) Schematic of three TCs for visual detection using CQDs-based test strips. Reproduced with permission from [39]. Copyright Nanoscale, 2018.
With the development of fluorescent immunosensor, the emerging CQDs has provided new ideas for the fabrication of economic and convenient immunoassay, potentially becoming an alternative and green fluorescent reagent [40]. Miao et al. reported the synthesis process of CQDs with blue fluorescent and its application as a sensing probe in the visual recognition and quantitative detection of three TCs [39] (Figure 3b). In this study, the LODs for TC, oxytetracycline (OTC) and chlortetracycline (CTC) are 5.18 nmol L−1, 6.06 nmol L−1 and 14 nmol L−1 respectively, which are lower than the obtained results in the above report [31]. The fluorescent signals of the three TC targets on the CQDs-based test strip are obviously different and can be distinguished visually, so that multiple targets in the one sample can be detected synchronously and sensitively.
2.2. Heavy Metal Ions
Heavy metal ions such as Fe3+, Cu2+, Fe2+, Ag+, Cr6+, Au3+, Hg2+ are an important aspect that cause the pollution of water or environment, which are easy to accumulate in animals and plants [41]. After entering the human body through the food chain, they can produce accumulated toxicity. It is of great significance to develop an effective, convenient and sensitive method for detecting heavy metal ions in foods [42][43][44]. Ming et al. synthesized a simple and low-cost N-CQDs (QY = 47.5%) via one-step hydrothermal method using thymidine as carbon source to fabricate a fluorescent sensor for sensitive detection of Cr6+ [45] (Figure 4A). Through the IFE, the obtained N-CQDs exhibited good fluorescent response to the target Cr6+. A good logarithm correlation between the fluorescent intensity of N-CQDs and the concentration of Cr6+ was obtained in the range of 0.1–430 µmol L−1 with R2 of 0.992 and low LOD of 1.26 nmol L−1. The fluorescent sensor can finish the detection process less than 1 min and has good repeatability, reproducibility and stability. Similarly, Lu et al. constructed a unique fluorescent 3D paper-based analysis device for Cr6+, in which applied blue N-CQDs as the fluorescent substrate [46]. In both above studies, the fluorescent quenching mechanism based on the IFE was used in the construction of different detection devices.
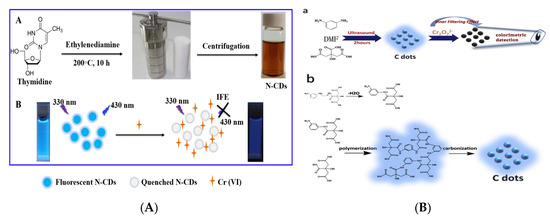
Figure 4. (A) Schematic of the detection of Cr6+ by N-CQDs fluorescent sensor. Reproduced with permission from [45]. Copyright Spectrochimica Acta Part A-molecular and Biomolecular Spectroscopy, 2019; (B) Schematic of the CQDs for the detection of Cr2O72− (a: the detection mechanism of Cr2O72−; b: the synthesis route of CQDs). Reproduced with permission from [47]. Copyright Dyes and Pigments, 2019.
Figure 4B has shown a dual-mode (fluorometric and colorimetric) CQDs fluorescent nano-sensing onsite platform for the detection of Cr2O72− in drinking water [47]. The water-soluble CQDs was prepared using a simple one-pot ultrasonic irradiation method, which had the excitation-dependent feature that the achieved emission evolved from blue (440 nm) to green luminescence (528 nm). The CQDs can be remarkably quenched by the chromate due to IFE and possesses highly selective and sensitive responses to Cr2O72− through the color changes at the same time. The dual sensing mode has established an effective assay for the recognition and detection of Cr2O72− with LODs as low as 0.14 nmol L−1 and 410 nmol L−1, respectively.
The CQDs-based test strips have obvious advantages in time-consuming and testing costs, which have a very broad development space in terms of rapid, sensitive and low-cost testing. One highly luminescent N-CQDs was synthesized by Raji et al. using jackfruit seeds as carbon source by a facile, green and rapid one-step microwave-assisted method, which displayed excellent water solubility, high QY (17.91%) and photo-stability, longer storage stability (stable up to >180 days without agglomeration) and low cytotoxicity [48]. The photoluminescence (PL) intensity of the resulting N-CQDs was linearly, selectively and sensitively quenched by Au3+ ions and the LOD of 239 nmol L−1 was obtained. Han et al. established a novel CQDs-based strategy for the ratiometric fluorescent detection of Cu2+ and glutathione (GSH) [49]. The fluorescent CQDs was firstly obtained through one-pot facile hydrothermal treatment using o-phenylenediamine (OPD) and citric acid as precursors. The oxidation product 2,3-diaminophenazine was obtained through the oxidation reaction of OPD and Cu2+, can not only emerge a new emission peak at 562 nm but also quench the fluorescent of CQDs at 446 nm (maximum emission) through FRET [50]. This ratiometric sensing system showed higher sensitivity toward Cu2+ in a range of 0.25–10.0 µmol L−1 with LOD of 0.076 µmol L−1 than the previous reports [51][52]. The Hg2+ has a strong affinity towards the carboxyl group [53]. Based on this, Hou et al. proposed a simple, economical and one-pot method to prepare functionalized fluorescent CQDs with good water solubility through electrochemical carbonization of sodium citrate and urea [54]. This CQDs with QY of 11.9% and average size of 2.4 nm were further applied as a label-free sensing probe for selective detection of Hg2+, achieving a LOD as low as 3.3 nmol L−1. Additionally, the easily functionalized surface of CQDs has facilitated the research and development of fluorescent sensing devices with specific functions and expanded the practical applications in the detection field. In the analysis of trace heavy metal ions, CQDs-based sensing probes have gradually become valuable sensing devices due to their high accuracy and reliability.
2.3. Mycotoxins
Mycotoxins produced by fungi such as molds are highly carcinogenic and toxic, easily contaminate in the foods and then enter the human body through food intake, which may even cause poisoning or death, seriously harming human health [55][56][57]. Therefore, the analysis and detection of mycotoxins in foods is an indispensable part of food safety detection. In addition to traditional large-scale instrument-based analysis and rapid immunoassays, the emerging CQDs-based sensing technology has provided new strategies for the detection of mycotoxins in foods [58][59][60]. Since mycotoxins exist in foods at trace amounts, CQDs are usually combined with MIP, while achieving the purification of complex matrix and the identification and detection of trace substance. Aflatoxin (AF) is classified as a highly toxic carcinogen by the World Health Organization (WHO) cancer research organization, which mainly pollutes the grain, oil and their products and seriously threatens human health [61]. Liang et al. prepared the fluorescent CQDs-coated dummy MIP (CQDs-DMIP) monolithic columns for pretreatment and further coupled with HPLC for selective recognition and detection of aflatoxin B1 (AFB1) in peanut [62]. The use of dummy template (5,7-dimethoxycoumarin) avoids the high toxicity and cost of AFB1. High enrichment factor over 71-fold and a LOD of 118 ng L−1 were obtained. The functional fluorescent sensor composed of monolithic column and HPLC integrates the identification, enrichment and detection process, which is superior to solid phase extraction (SPE)-HPLC coupled with ultraviolet (UV) on selectivity and sensitivity [63][64]. Guo et al. also used a dummy template 2-oxindole to electrodeposit a MIP membrane on the glassy carbon electrode (GCE) to fabricate one electrochemical sensor (MIP-Au/CS-CQDs/GCE) for the detection of patulin in fresh apple juice [65]. Figure 5a has shown the preparation process of MIP-Au/CS-CQDs/GCE and the detection process of patulin. The CQDs and chitosan (CS) modified on the surface of GCE are designed to improve the electron-transfer rate, expand the electroactive surface of the electrode and enhance the signal strength. The sensing system composed of hybrid composites CS-CQDs and MIP-Au has been demonstrated to be a new strategy for the detection of patulin with a lower LOD of 7.57 × 10−13 mol L−1. Shao et al. developed a molecularly imprinted fluorescent quenching particles by encapsulating silicon-based CQDs in MIPs material for sensitive detection of zearalenone (ZEN) in corn, which achieved a lower LOD (0.02 mg L−1) [66].
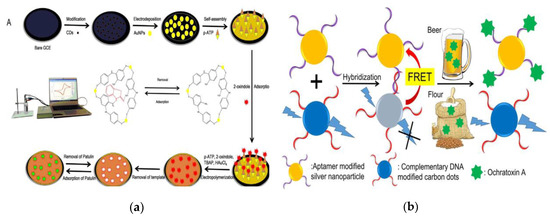
Figure 5. (a) Schematic of the preparation process of MIP-Au/CS-CQDs/GCE on glassy carbon electrode (GCE) and the detection of patulin. Reproduced with permission from [65]. Copyright Biosensors & Bioelectronics, 2017; (b) Schematic of fluorescent detection of ochratoxin A (OTA) in beer and flour based on FRET. Reproduced with permission from [67]. Copyright Talanta, 2018.
Immunochromatographic test strips (ICTS) have unparalleled advantages in rapid, low-cost testing and screening of large numbers of samples [68]. With the help of the fluorescent properties of CQDs, fluorescent ICTS can be developed for the analysis and detection of mycotoxins. Li et al. have reported an innovative visually “turn-off” design of fluorescent lateral flow immunochromatographic assays (FLFIAs) for the semi-quantitative detection of ZEN in cereals based on the characteristic of FRET [69]. Wang et al. also designed a FRET system using N-CQDs as energy donor, DNA and 6-mercapto-1-hexanol modified AgNPs as energy acceptor for the quick detection of ochratoxin A (OTA) in agricultural products [67] (Figure 5b). The introduction of complementary DNA, aptamers and AgNPs makes the FRET system have good sensing response in a wide concentration range to OTA (10–5000 nmol L−1). The distance-sensitive FRET and the tail-tail arrangement of DNA strands make the detection procedure more sensitive and can be finished within 13 min.
2.4. Food Additives
In the food industry, food additives play an important role in ensuring the color and flavor of food and improving the quality of food. However, the excessive use of additives (colorants, coagulants, preservatives, etc.) and the addition of illegal additives (Sudan I, melamine, clenbuterol, etc.) have caused new food safety issues and posed a serious threat to human health [70][71]. Sudan I is one colorant that is banned in foods in many countries but because of its bright color and low price, many manufacturers still use it illegally in the process of food production. It is of great significance to develop sensitive, convenient and effective strategies for Sudan I analysis in foods [72]. Su et al. successfully synthesized CQDs with QY of 14% through a simple, low-cost and green hydrothermal treatment using cigarette filters as carbon source, which showed a strong emission at the wavelength of 465 nm with an optimum excitation of 365 nm [73]. A sensing platform for the detection of Sudan I was further designed using this controllable quenching fluorescent CQDs in chili and tomato samples. This fluorescent probe has been testified that possesses high selectivity and sensitivity (LOD: 0.95 µmol L−1). Melamine is an illegal additive added to milk to improve the content of protein (key reference indicator to evaluate the quality and safety of milk products) [74]. Hu et al. have designed one Au@CQDs nanocomposite for analyzing melamine in milk visually combined with a smartphone [75]. Figure 6A has shown the scheme of fluorescent assay composed of Au@CQDs for this sensing process with a lower LOD of 3.6 nmol L−1, obtaining good recoveries (105.64–102.75%) in the range of 1–10 µmol L−1. In such a detection system, the combination of fluorescent spectrum and the portable devices realizes fast, simple and visual detection, providing a new direction for the development of intelligent detection methods. Rhodamine 6G (R6G), as a colorant, is forbidden to use in the process of food production. Cui et al. have employed the CQDs-embedded periodic mesoporous organosilica (PMO) as the support for designing an MIP sensor (CQDs-PMO-MIS) for highly sensitive detection of R6G [76]. The synergistic effect of PMO, CQDs and MIPs achieves sensitive and stable detection of R6G in the concentration range of 4–7 mg L−1 and the PMO provides highly selective recognition sites for the template, providing a useful reference for the design of novel MIP sensors. Xu et al. reported the green synthetic process of CQDs by hydrothermal treatment of fresh aloe and used it as a fluorescent probe for sensitive and selective detection of tartrazine in candy, steamed bread and honey [77]. The N, Cl-doped fluorescent CQDs (N/Cl-CQDs) with a QY of 60.52% synthesized by Yang et al. was applied for the detection of tartrazine in beverages [78]. The doped N, Cl atoms in CQDs have adjusted the band gap of semiconductor [79], resulting in the obvious improvement of fluorescent and surface physicochemical properties compared to pure CQDs, which is verified in the fluorescent QY and the LOD via analyzing multiple reports.
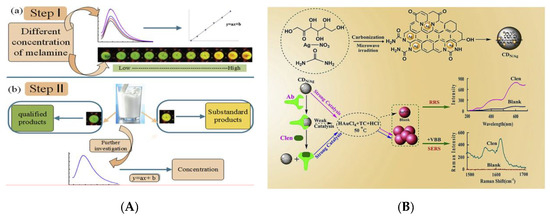
Figure 6. (A) Schematic of different response and the detection of Au@CQDs-based to melamine. Reproduced with permission from [75]. Copyright Food Chemistry, 2019; (B) Schematic of the formation of N/Ag-CQDs and the principle of the catalytic detection of Clen. Reproduced with permission from [80]. Copyright Talanta, 2020.
Clenbuterol (Clen), also called “lean meat powder,” has an obvious effect in reducing fat content and increasing lean meat rate to the animals. The Clen was illegally added into the animal feeds, causing serious threat to human health. In order to control the use of Clen and evaluate the quality of meat products, a multifunctional N, Ag-doped CQDs (N/Ag-CQDs) was synthesized by Yao and applied to construct the dual-spectroscopic immunosensor for quantitative analysis and detection of Clen [80] (Figure 6B). Based on surface-enhanced Raman scattering (SERS) and resonance Rayleigh scattering (RRS) of N/Ag-CQDs, a low LOD of 0.68 ng L−1 was obtained without the fluorescent labeling. Yang et al. have prepared a branched polyethyleneimine-functionalized CQDs (BPEI-CQDs) via a microwave-assisted process, which was further applied as a fluorescent probe to detect tannic acid (TA) in white wine and obtained a remarkable sensitivity [81]. In the synthesis of the BPEI-CQDs composite, the passivation of BPEI induces the CQDs to generate recognizable active sites on the surface, thereby improving the targeted recognition ability for the molecules. The hydrophilic groups are lied on the surface of functionalized CQDs, making it suitable for fluorescent sensing detection.
As a new carbon-based nanomaterial, although the research of CQDs applied in food additive detection is less, the techniques using CQDs as fluorescent sensing probe or combined with fluorescent spectrometry have a broad application prospect in the detection of food additives, which is expected to become a new detection strategy with convenience and benefit.
References
- Jun Zuo; Tao Jiang; Xiaojing Zhao; Xiaohong Xiong; Saijin Xiao; Zhiqiang Zhu; Preparation and Application of Fluorescent Carbon Dots. Journal of Nanomaterials 2015, 2015, 1-13, 10.1155/2015/787862.
- Meng-Li Liu; Bin-Bin Chen; Chun-Mei Li; Chengzhi Huang; Carbon dots prepared for fluorescence and chemiluminescence sensing. Science China Chemistry 2019, 62, 968-981, 10.1007/s11426-019-9449-y.
- Peng Miao; Kun Han; Yuguo Tang; Bidou Wang; Tao Lin; Wenbo Cheng; Recent advances in carbon nanodots: synthesis, properties and biomedical applications. Nanoscale 2015, 7, 1586-1595, 10.1039/c4nr05712k.
- Song-Ling Ye; Jia-Jia Huang; Lin Luo; Hui-Jun Fu; Yuanming Sun; Yu-Dong Shen; Hongtao Lei; Zhenlin Xu; Preparation of Carbon Dots and Their Application in Food Analysis as Signal Probe. Chinese Journal of Analytical Chemistry 2017, 45, 1571-1581, 10.1016/S1872-2040(17)61045-4.
- Xueli Luo; Yong Han; Xiumei Chen; Wenzhi Tang; Tianli Yue; Zhonghong Li; Carbon dots derived fluorescent nanosensors as versatile tools for food quality and safety assessment: A review. Trends in Food Science & Technology 2020, 95, 149-161, 10.1016/j.tifs.2019.11.017.
- Lomme J. Deleu; Edith Wilderjans; Ingrid Van Haesendonck; Christophe M. Courtin; Kristof Brijs; Jan A. Delcour; Storage induced conversion of ovalbumin into S-ovalbumin in eggs impacts the properties of pound cake and its batter. Food Hydrocolloids 2015, 49, 208-215, 10.1016/j.foodhyd.2015.03.014.
- Xuan Fu; Long Sheng; Yisha Yu; Meihu Ma; Zhaoxia Cai; Xi Huang; Rapid and universal detection of ovalbumin based on N,O,P-co-doped carbon dots-fluorescence resonance energy transfer technology. Sensors and Actuators B: Chemical 2018, 269, 278-287, 10.1016/j.snb.2018.04.134.
- Rahul Purbia; Santanu Paria; A simple turn on fluorescent sensor for the selective detection of thiamine using coconut water derived luminescent carbon dots. Biosensors and Bioelectronics 2016, 79, 467-475, 10.1016/j.bios.2015.12.087.
- Shao-Ping Li; C.F. Qiao; Y.W. Chen; J. Zhao; X.M. Cui; Q.W. Zhang; X.M. Liu; D.J. Hu; A novel strategy with standardized reference extract qualification and single compound quantitative evaluation for quality control of Panax notoginseng used as a functional food. Journal of Chromatography A 2013, 1313, 302-307, 10.1016/j.chroma.2013.07.025.
- Hsin-Lan Liu; Tsai-Hua Kao; Chyuan-Yuan Shiau; Bing-Huei Chen; Functional components in Scutellaria barbata D. Don with anti-inflammatory activity on RAW 264.7 cells. Journal of Food and Drug Analysis 2018, 26, 31-40, 10.1016/j.jfda.2016.11.022.
- Huan Yang; Liu Yang; Yusheng Yuan; Shuang Pan; Jidong Yang; Jingjing Yan; Hui Zhang; Qianqian Sun; Xiaoli Hu; A portable synthesis of water-soluble carbon dots for highly sensitive and selective detection of chlorogenic acid based on inner filter effect. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2018, 189, 139-146, 10.1016/j.saa.2017.07.065.
- Shelja Sharma; Ahmad Umar; Swati Sood; Surinder Kumar Mehta; Sushil Kumar Kansal; Photoluminescent C-dots: An overview on the recent development in the synthesis, physiochemical properties and potential applications. Journal of Alloys and Compounds 2018, 748, 818-853, 10.1016/j.jallcom.2018.03.001.
- Frantisek Kvasnicka; Jana Čopíková; Rudolf Ševčík; Jana Krátká; Andrej Syntytsia; Michal Voldřich; Determination of phenolic acids by capillary zone electrophoresis and HPLC. Open Chemistry 2008, 6, 410-418, 10.2478/s11532-008-0032-5.
- Jiayu Zhang; Zijian Wang; Yun Li; Ying Liu; Wei Cai; Chen Li; Jian-Qiu Lu; Yan-Jiang Qiao; A strategy for comprehensive identification of sequential constituents using ultra-high-performance liquid chromatography coupled with linear ion trap–Orbitrap mass spectrometer, application study on chlorogenic acids in Flos Lonicerae Japonicae. Talanta 2016, 147, 16-27, 10.1016/j.talanta.2015.09.039.
- Maria Mantzorou; Eleni Pavlidou; George Vasios; Eftychia Tsagalioti; Constantinos Giaginis; Effects of curcumin consumption on human chronic diseases: A narrative review of the most recent clinical data. Phytotherapy Research 2018, 32, 957-975, 10.1002/ptr.6037.
- Shanshan Zhang; Jun Zou; Peiyang Li; Xiumei Zheng; Dan Feng; Curcumin Protects against Atherosclerosis in Apolipoprotein E-Knockout Mice by Inhibiting Toll-like Receptor 4 Expression. Journal of Agricultural and Food Chemistry 2018, 66, 449-456, 10.1021/acs.jafc.7b04260.
- Yang Liu; Xiaojuan Gong; Wenjuan Dong; Ruixin Zhou; Shaomin Shuang; Chuan Dong; Nitrogen and phosphorus dual-doped carbon dots as a label-free sensor for Curcumin determination in real sample and cellular imaging. Talanta 2018, 183, 61-69, 10.1016/j.talanta.2018.02.060.
- Ganesan Muthusankar; Murugan Sethupathi; Shen-Ming Chen; Ramadhass Keerthika Devi; Rajendran Vinoth; G. Gopu; Narayanasamy Anandhan; Nallathambi Sengottuvelan; N-doped carbon quantum dots @ hexagonal porous copper oxide decorated multiwall carbon nanotubes: A hybrid composite material for an efficient ultra-sensitive determination of caffeic acid. Composites Part B: Engineering 2019, 174, 106973, 10.1016/j.compositesb.2019.106973.
- Longhua Xu; Mingfei Pan; Guozhen Fang; Shuo Wang; Carbon dots embedded metal-organic framework@molecularly imprinted nanoparticles for highly sensitive and selective detection of quercetin. Sensors and Actuators B: Chemical 2019, 286, 321-327, 10.1016/j.snb.2019.01.156.
- Xuemei Zhou; Zhimin Tian; Jing Li; Hong Ruan; Yuanyuan Ma; Zhi Yang; Yongquan Qu; Synergistically enhanced activity of graphene quantum dot/multi-walled carbon nanotube composites as metal-free catalysts for oxygen reduction reaction. Nanoscale 2014, 6, 2603-2607, 10.1039/c3nr05578g.
- Raji Atchudan; Nallal Muthuchamy; Thomas Nesakumar Jebakumar Immanuel Edison; Suguna Perumal; Rajangam Vinodh; Kang Hyun Park; Yong Rok Lee; An ultrasensitive photoelectrochemical biosensor for glucose based on bio-derived nitrogen-doped carbon sheets wrapped titanium dioxide nanoparticles. Biosensors and Bioelectronics 2019, 126, 160-169, 10.1016/j.bios.2018.10.049.
- B. Sinduja; S. Abraham John; Sensitive determination of rutin by spectrofluorimetry using carbon dots synthesized from a non-essential amino acid. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2018, 193, 486-491, 10.1016/j.saa.2017.12.067.
- Jia-Wei Zhou; Xue-Mei Zou; Shang-Hong Song; Guan-Hua Chen; Quantum Dots Applied to Methodology on Detection of Pesticide and Veterinary Drug Residues. Journal of Agricultural and Food Chemistry 2018, 66, 1307-1319, 10.1021/acs.jafc.7b05119.
- Shanli Yang; Jiesheng Liang; Shenglian Luo; Chengbin Liu; Yanhong Tang; Supersensitive Detection of Chlorinated Phenols by Multiple Amplification Electrochemiluminescence Sensing Based on Carbon Quantum Dots/Graphene. Analytical Chemistry 2013, 85, 7720-7725, 10.1021/ac400874h.
- Mingfei Pan; Zongjia Yin; Kaixin Liu; Xiaoling Du; Huilin Liu; Shuo Wang; Carbon-Based Nanomaterials in Sensors for Food Safety. Nanomaterials 2019, 9, 1330, 10.3390/nano9091330.
- Dianwei Zhang; Jiaqi Tang; Huilin Liu; Rapid determination of lambda-cyhalothrin using a fluorescent probe based on ionic-liquid-sensitized carbon dots coated with molecularly imprinted polymers. Zeitschrift für Analytische Chemie 2019, 411, 5309-5316, 10.1007/s00216-019-01912-0.
- Lingxin Chen; Xiaoyan Wang; Wenhui Lu; Xiaqing Wu; Jinhua Li; Molecular imprinting: perspectives and applications. Chemical Society Reviews 2016, 45, 2137-2211, 10.1039/c6cs00061d.
- Mao Wu; Yajun Fan; Jiawei Li; Danqing Lu; Yaping Guo; Lianwu Xie; Yiqiang Wu; Vinyl Phosphate-Functionalized, Magnetic, Molecularly-Imprinted Polymeric Microspheres' Enrichment and Carbon Dots' Fluorescence-Detection of Organophosphorus Pesticide Residues. Polymers 2019, 11, 1770, 10.3390/polym11111770.
- Yanzhao Fu; Shaojing Zhao; Shuilin Wu; Li Huang; Ting Xu; Xuejian Xing; Minhuan Lan; Xiangzhi Song; A carbon dots-based fluorescent probe for turn-on sensing of ampicillin. Dyes and Pigments 2020, 172, 107846, 10.1016/j.dyepig.2019.107846.
- Phannika Raksawong; Piyaluk Nurerk; Kochaporn Chullasat; Proespichaya Kanatharana; Opas Bunkoed; A polypyrrole doped with fluorescent CdTe quantum dots and incorporated into molecularly imprinted silica for fluorometric determination of ampicillin. Mikrochimica Acta 2019, 186, 338, 10.1007/s00604-019-3447-0.
- Huiyu Li; Li Zhao; Yuan Xu; Tianyu Zhou; Haochi Liu; Ning Huang; Jie Ding; Yi Li; Lan Ding; Single-hole hollow molecularly imprinted polymer embedded carbon dot for fast detection of tetracycline in honey. Talanta 2018, 185, 542-549, 10.1016/j.talanta.2018.04.024.
- Juan Hou; Huiyu Li; Long Wang; Ping Zhang; Tianyu Zhou; Hong Ding; Lan Ding; Rapid microwave-assisted synthesis of molecularly imprinted polymers on carbon quantum dots for fluorescent sensing of tetracycline in milk. Talanta 2016, 146, 34-40, 10.1016/j.talanta.2015.08.024.
- Ashraf M. Mahmoud; Mohamed M. El-Wekil; Mater H. Mahnashi; Marwa F. B. Ali; Saad A. Alkahtani; Modification of N,S co-doped graphene quantum dots with p-aminothiophenol-functionalized gold nanoparticles for molecular imprint-based voltammetric determination of the antiviral drug sofosbuvir. Mikrochimica Acta 2019, 186, 617, 10.1007/s00604-019-3647-7.
- Shuai Chen; Xin Hai; Xu-Wei Chen; Jian-Hua Wang; In Situ Growth of Silver Nanoparticles on Graphene Quantum Dots for Ultrasensitive Colorimetric Detection of H2O2 and Glucose. Analytical Chemistry 2014, 86, 6689-6694, 10.1021/ac501497d.
- Purnendu Kumar Pathak; Anil Kumar; Bhim Bali Prasad; Functionalized nitrogen doped graphene quantum dots and bimetallic Au/Ag core-shell decorated imprinted polymer for electrochemical sensing of anticancerous hydroxyurea. Biosensors and Bioelectronics 2018, 127, 10-18, 10.1016/j.bios.2018.11.055.
- Mingfei Pan; Jingying Yang; Kaixin Liu; Zongjia Yin; Tianyu Ma; Shengmiao Liu; Longhua Xu; Shuo Wang; Liu; Noble Metal Nanostructured Materials for Chemical and Biosensing Systems. Nanomaterials 2020, 10, 209, 10.3390/nano10020209.
- Yuangen Wu; Yuangen Wu; Pei Zhou; Wenping Yang; Han Tao; Shuyi Qiu; Caiwei Feng; A novel fluorescent aptasensor for ultrasensitive and selective detection of acetamiprid pesticide based on the inner filter effect between gold nanoparticles and carbon dots. The Analyst 2018, 143, 5151-5160, 10.1039/c8an01166d.
- Tianrong Zhan; Guiyan Ding; Wei Cao; Jiamin Li; Xilin She; Hongni Teng; Amperometric sensing of catechol by using a nanocomposite prepared from Ag/Ag2O nanoparticles and N,S-doped carbon quantum dots. Mikrochimica Acta 2019, 186, 743, 10.1007/s00604-019-3848-0.
- Hong Miao; Yingyi Wang; XiaoMing Yang; Carbon dots derived from tobacco for visually distinguishing and detecting three kinds of tetracyclines. Nanoscale 2018, 10, 8139-8145, 10.1039/c8nr02405g.
- Cui Liu; Dianhua Ning; Cheng Zhang; ZhengJie Liu; Ruilong Zhang; Jun Zhao; Tingting Zhao; Bianhua Liu; Zhongping Zhang; Dual-Colored Carbon Dot Ratiometric Fluorescent Test Paper Based on a Specific Spectral Energy Transfer for Semiquantitative Assay of Copper Ions. ACS Applied Materials & Interfaces 2017, 9, 18897-18903, 10.1021/acsami.7b05827.
- Haibin Tang; Chuhong Zhu; Guowen Meng; Nianqiang Wu; Review—Surface-Enhanced Raman Scattering Sensors for Food Safety and Environmental Monitoring. Journal of The Electrochemical Society 2018, 165, B3098-B3118, 10.1149/2.0161808jes.
- Yawen Li; Yuzhi Chen; Hao Yu; Limei Tian; Zhuo Wang; Portable and smart devices for monitoring heavy metal ions integrated with nanomaterials. TrAC Trends in Analytical Chemistry 2018, 98, 190-200, 10.1016/j.trac.2017.11.011.
- Mojtaba Shamsipur; Karam Molaei; Fatemeh Molaabasi; Saman Hosseinkhanid; Naader Alizadeh; Mohsen Aipour; Soroush Moassess; One-step synthesis and characterization of highly luminescent nitrogen and phosphorus co-doped carbon dots and their application as highly selective and sensitive nanoprobes for low level detection of uranyl ion in hair and water samples and application to cellular imaging. Sensors and Actuators B: Chemical 2018, 257, 772-782, 10.1016/j.snb.2017.11.018.
- Shan Huang; Erli Yang; Jiandong Yao; Xu Chu; Yi Liu; Qi Xiao; Nitrogen, phosphorus and sulfur tri-doped carbon dots are specific and sensitive fluorescent probes for determination of chromium(VI) in water samples and in living cells. Mikrochimica Acta 2019, 186, 851, 10.1007/s00604-019-3941-4.
- Fanglin Ming; Jingzhou Hou; Danqun Huo; Mei Yang; Xianfeng Wang; Jiawei Li; Danqun Huo; Qiang He; One-step synthesized fluorescent nitrogen doped carbon dots from thymidine for Cr (VI) detection in water. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2019, 222, 117165, 10.1016/j.saa.2019.117165.
- Kun-Hua Lu; Jia-Hui Lin; Cheng-Yu Lin; Chien-Fu Chen; Yi-Chun Yeh; A fluorometric paper test for chromium(VI) based on the use of N-doped carbon dots. Mikrochimica Acta 2019, 186, 227, 10.1007/s00604-019-3337-5.
- Gongxi Qiao; Ng Lu; Yiping Tang; Jinwei Gao; Qianming Wang; Smart choice of carbon dots as a dual-mode onsite nanoplatform for the trace level detection of Cr2O72-. Dyes and Pigments 2019, 163, 102-110, 10.1016/j.dyepig.2018.11.049.
- Kaviyarasan Raji; Vadivel Ramanan; Perumal Ramamurthy; Ramanan Vadivel; Facile and green synthesis of highly fluorescent nitrogen-doped carbon dots from jackfruit seeds and its applications towards the fluorimetric detection of Au3+ ions in aqueous medium and in in vitro multicolor cell imaging. New Journal of Chemistry 2019, 43, 11710-11719, 10.1039/c9nj02590a.
- Zhu Han; Danyang Nan; Huan Yang; Qianqian Sun; Shuang Pan; Hui Liu; Xiaoli Hu; Carbon quantum dots based ratiometric fluorescence probe for sensitive and selective detection of Cu2+ and glutathione. Sensors and Actuators B: Chemical 2019, 298, 126842, 10.1016/j.snb.2019.126842.
- Fanlin Zu; Fanyong Yan; Zhangjun Bai; Jinxia Xu; Yinyin Wang; Yicun Huang; Xuguang Zhou; The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Mikrochimica Acta 2017, 184, 1899-1914, 10.1007/s00604-017-2318-9.
- Xi-Ke Tian; Xike Tian; Chao Yang; Yong Li; Zhaoxin Zhou; Yanxin Wang; Fang Xiang; Highly selective and sensitive determination of copper ion based on a visual fluorescence method. Sensors and Actuators B: Chemical 2017, 240, 66-75, 10.1016/j.snb.2016.08.155.
- Jing Xu; Zuokai Wang; Caiyun Liu; Zhenghe Xu; Baocun Zhu; Ning Wang; Kun Wang; Jiangting Wang; A Colorimetric and Fluorescent Probe for the Detection of Cu2+ in a Complete Aqueous Solution. Analytical Sciences 2018, 34, 453-457, 10.2116/analsci.17p517.
- Wenbo Lu; Xiaoyun Qin; Sen Liu; Guohui Chang; Yingwei Zhang; Yonglan Luo; Abdullah M. Asiri; Abdulrahman O. Al-Youbi; Xuping Sun; Economical, Green Synthesis of Fluorescent Carbon Nanoparticles and Their Use as Probes for Sensitive and Selective Detection of Mercury(II) Ions. Analytical Chemistry 2012, 84, 5351-5357, 10.1021/ac3007939.
- Yuxin Hou; Qiujun Lu; Jianhui Deng; Haitao Li; Youyu Zhang; One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Analytica Chimica Acta 2015, 866, 69-74, 10.1016/j.aca.2015.01.039.
- MW,Trucksess. Mycotoxins; Journal of AOAC International 1998, 81, 128–137.
- H.A. Wall-Martínez; A. Ramírez-Martínez; N. Wesolek; C. Brabet; N. Durand; G. C. Rodríguez-Jimenes; M. A. García-Alvarado; M. A. Salgado-Cervantes; V. Robles-Olvera; Alain-Claude Roudot; et al. Risk assessment of exposure to mycotoxins (aflatoxins and fumonisins) through corn tortilla intake in Veracruz City (Mexico). Food Additives & Contaminants: Part A 2019, 36, 929-939, 10.1080/19440049.2019.1588997.
- S.A. Tittlemier; B. Cramer; C. Dall’Asta; M.H. Iha; V.M.T. Lattanzio; C. Maragos; M. Solfrizzo; M. Stranska; J. Stroka; M. Sumarah; et al. Developments in mycotoxin analysis: an update for 2018-19. World Mycotoxin Journal 2020, 13, 3-24, 10.3920/wmj2019.2535.
- Henry Fischbach; Joseph Y Rodricks; Current Efforts of the Food and Drug Administration to Control Mycotoxins in Food. Journal of AOAC INTERNATIONAL 1973, 56, 767-770, 10.1093/jaoac/56.3.767.
- Ruben R.G. Soares; Alessandra Ricelli; Corrado Fanelli; Domenico Caputo; Giampiero De Cesare; Virginia Chu; Maria R. Aires‐Barros; João Pedro Conde; Advances, challenges and opportunities for point-of-need screening of mycotoxins in foods and feeds. The Analyst 2018, 143, 1015-1035, 10.1039/c7an01762f.
- Fuguo Xing; Haibo Yao; Yang Liu; Xiaofeng Dai; Robert L. Brown; Deepak Bhatnagar; Recent developments and applications of hyperspectral imaging for rapid detection of mycotoxins and mycotoxigenic fungi in food products. Critical Reviews in Food Science and Nutrition 2017, 59, 173-180, 10.1080/10408398.2017.1363709.
- Ismail Y.S. Rustom; Aflatoxin in food and feed: occurrence, legislation and inactivation by physical methods. Food Chemistry 1997, 59, 57-67, 10.1016/s0308-8146(96)00096-9.
- GuoHuan Liang; Haiyun Zhai; Lu Huang; Xuecai Tan; Qing Zhou; Xiao Yu; Haidan Lin; Synthesis of carbon quantum dots-doped dummy molecularly imprinted polymer monolithic column for selective enrichment and analysis of aflatoxin B 1 in peanut. Journal of Pharmaceutical and Biomedical Analysis 2018, 149, 258-264, 10.1016/j.jpba.2017.11.012.
- Maryam Vosough; Mahin Bayat; Amir Salemi; Matrix-free analysis of aflatoxins in pistachio nuts using parallel factor modeling of liquid chromatography diode-array detection data. Analytica Chimica Acta 2010, 663, 11-18, 10.1016/j.aca.2010.01.039.
- Mashaallah Rahmani; Elham Ghasemi; Mojtaba Sasani; Application of response surface methodology for air assisted-dispersive liquid- liquid microextraction of deoxynivalenol in rice samples prior to HPLC-DAD analysis and comparison with solid phase extraction cleanup. Talanta 2017, 165, 27-32, 10.1016/j.talanta.2016.12.031.
- Wei Guo; Fuwei Pi; Hongxia Zhang; Jiadi Sun; Yinzhi Zhang; Xiulan Sun; A novel molecularly imprinted electrochemical sensor modified with carbon dots, chitosan, gold nanoparticles for the determination of patulin. Biosensors and Bioelectronics 2017, 98, 299-304, 10.1016/j.bios.2017.06.036.
- Manyu Shao; Ming Yao; Sarah De Saeger; Liping Yan; Suquan Song; Carbon Quantum Dots Encapsulated Molecularly Imprinted Fluorescence Quenching Particles for Sensitive Detection of Zearalenone in Corn Sample. Toxins 2018, 10, 438, 10.3390/toxins10110438.
- Chengke Wang; Rong Tan; Dan Chen; Fluorescence method for quickly detecting ochratoxin A in flour and beer using nitrogen doped carbon dots and silver nanoparticles. Talanta 2018, 182, 363-370, 10.1016/j.talanta.2018.02.007.
- Mingfei Pan; Tianyu Ma; Jingying Yang; Shijie Li; Shengmiao Liu; Shuo Wang; Development of Lateral Flow Immunochromatographic Assays Using Colloidal Au Sphere and Nanorods as Signal Marker for the Determination of Zearalenone in Cereals. Foods 2020, 9, 281, 10.3390/foods9030281.
- Shijie Li; Junping Wang; Wei Sheng; Wenjun Wen; Ying Gu; Shuo Wang; Fluorometric lateral flow immunochromatographic zearalenone assay by exploiting a quencher system composed of carbon dots and silver nanoparticles. Mikrochimica Acta 2018, 185, 388, 10.1007/s00604-018-2916-1.
- Jia-Huan Qu; Qingyi Wei; Da-Wen Sun; Carbon dots: Principles and their applications in food quality and safety detection. Critical Reviews in Food Science and Nutrition 2018, 58, 2466-2475, 10.1080/10408398.2018.1437712.
- Man Lv; Yang Liu; Jinhui Geng; Xiaohong Kou; Zhihong Xin; Dayong Yang; Engineering nanomaterials-based biosensors for food safety detection. Biosensors and Bioelectronics 2018, 106, 122-128, 10.1016/j.bios.2018.01.049.
- Fernanda C.O.L. Martins; Michelle A. Sentanin; Djenaine De Souza; Analytical methods in food additives determination: Compounds with functional applications. Food Chemistry 2019, 272, 732-750, 10.1016/j.foodchem.2018.08.060.
- Su Anmei; Zhong Qingmei; Chen Yuye; Yilin Wang; Preparation of carbon quantum dots from cigarette filters and its application for fluorescence detection of Sudan I. Analytica Chimica Acta 2018, 1023, 115-120, 10.1016/j.aca.2018.03.024.
- Qi Li; Pan Song; Jian Guo Wen; Melamine and food safety: a 10-year review. Current Opinion in Food Science 2019, 30, 79-84, 10.1016/j.cofs.2019.05.008.
- Xuetao Hu; Jiyong Shi; Yongqiang Shi; Xiaobo Zou; Muhammad Arslan; Wen Zhang; Xiaowei Huang; Zhihua Li; Yiwei Xu; Use of a smartphone for visual detection of melamine in milk based on Au@Carbon quantum dots nanocomposites. Food Chemistry 2019, 272, 58-65, 10.1016/j.foodchem.2018.08.021.
- Chuanfeng Cui; Juying Lei; Lingang Yang; Bin Shen; Lingzhi Wang; Jinlong Zhang; Carbon-dot-encapsulated molecularly imprinted mesoporous organosilica for fluorescent sensing of rhodamine 6G. Research on Chemical Intermediates 2018, 44, 4633-4640, 10.1007/s11164-018-3279-2.
- Hua Xu; Xiupei Yang; Gu Li; Chuan Zhao; Xiangjun Liao; Green Synthesis of Fluorescent Carbon Dots for Selective Detection of Tartrazine in Food Samples. Journal of Agricultural and Food Chemistry 2015, 63, 6707-6714, 10.1021/acs.jafc.5b02319.
- Xiupei Yang; Jing Xu; Na Luo; Fenglin Tang; Maoxue Zhang; Bin Zhao; N,Cl co-doped fluorescent carbon dots as nanoprobe for detection of tartrazine in beverages. Food Chemistry 2020, 310, 125832, 10.1016/j.foodchem.2019.125832.
- Yan Gong; Jie Zhao; Small Carbon Quantum Dots, Large Photosynthesis Enhancement. Journal of Agricultural and Food Chemistry 2018, 66, 9159-9161, 10.1021/acs.jafc.8b01788.
- Dongmei Yao; Chongning Li; Guiqing Wen; Aihui Liang; Zhi-Liang Jiang; A highly sensitive and accurate SERS/RRS dual-spectroscopic immunosensor for clenbuterol based on nitrogen/silver-codoped carbon dots catalytic amplification. Talanta 2020, 209, 120529, 10.1016/j.talanta.2019.120529.
- Pei Yang; Ziqi Zhu; Minzhi Chen; Yizhong Cao; Weimin Chen; Microwave-assisted synthesis of polyamine-functionalized carbon dots from xylan and their use for the detection of tannic acid. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2019, 213, 301-308, 10.1016/j.saa.2019.01.043.




