
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | zhang wenrui | + 1261 word(s) | 1261 | 2021-05-19 07:12:18 | | | |
| 2 | Lindsay Dong | Meta information modification | 1261 | 2021-05-20 04:09:03 | | |
Video Upload Options
Asparagine endopeptidase (AEP), also called legumain, is currently the only known cysteine protease that specifically cleaves peptide bonds in asparaginyl residue in the mammalian genome. Since 2003, AEP has been reported to be widely expressed in a variety of carcinomas and is considered a potential therapeutic target. In the following years, researchers intensively investigated the substrates of AEP and the mechanism of AEP in partial tumors. With the identification of substrate proteins such as P53, integrin αvβ3, MMP-2, MMP-9, the biochemical mechanism of AEP in carcinomas is also more precise. This review will clarify the probable mechanisms of AEP in the progression of breast carcinoma, glioblastoma, gastric carcinoma, and epithelial ovarian carcinoma. This review will also discuss the feasibility of targeted therapy with AEP inhibitor (AEPI) in these carcinomas.
1. Introduction
Asparagine endopeptidase (AEP) is a cysteine protease that was initially discovered in leguminous seeds in the early 1980s and was first isolated and identified in mammals in 1997 [1][2] (Figure 1). AEP predominantly exists in the late endosomes and lysosomes. The process of activation in vitro is well understood. There are several cysteine protease inhibitors in mammals; however, only the family 2 cystatins were able to inhibit AEP.
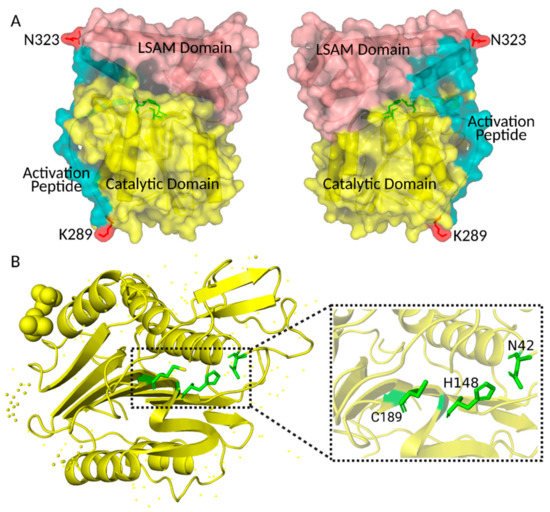
Figure 1. Crystal structure of human pro-AEP and AEP. (A) Crystal structure of human pro-AEP. The caspase-like catalytic domain is shown in yellow, the Activation Peptide in blue and the LSAM domain in pink; the C-terminal processing sites K289 and N323 are shown in red. (B) Crystal structure of human AEP. The active site residues (Asn42, His148, and Cys189) of the catalytic domain are shown in green. The figure was created using PYMOL.
2. Glioblastoma
AEP Promotes Glioblastoma Progression by Blocking the Tumor-Suppressive Function of P53 Protein (Figure 2).
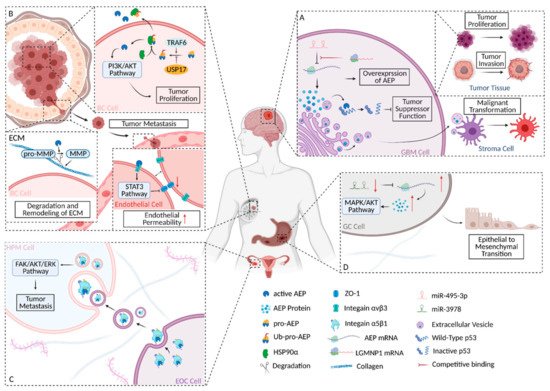
Figure 2. Schematic diagram of AEP’s roles in several carcinomas. (A) The biochemical and regulation mechanism of AEP in GBM. (B) The biochemical and regulation mechanism of AEP in BC. (C) Schematic diagram of the biochemical mechanism and involved signaling pathway of AEP in EOC. (D) Schematic diagram of the involved signaling pathway and regulation mechanism of AEP in GC. The figure was created using BioRender.com.
Preliminary validation of AEP as an effective target for tumor therapy was achieved with AEPI, which is the most promising chemotherapeutic agent for targeting AEP and AEP silencing by shRNA. Inhibition of AEP’s function can significantly suppress tumor progression and prolong the survival time of mice [3]. Due to its nature as a small molecule and its specificity, AEPI can effectively cross the blood–brain barrier (BBB) and has a promising future as a chemotherapeutic agent for the treatment of GBM.
3. Breast Carcinoma
In BC cells, TRAF6 directly catalyzed K63-linked poly-ubiquitination of pro-AEP; this process could be reversed by USP17 [4]. Subsequently, heat shock protein 90-α (HSP90α), a stress-inducible molecular chaperone [5], was recruited by ubiquitinated pro-AEP to assemble complexes. The complexes enabled the maintenance of stabilization of pro-AEP and promoted the secretion of pro-AEP into the extracellular matrix under stress conditions [4]. On the one hand, stabilized pro-AEP can maintain a stable concentration of AEP in BC cells, thus playing a role in BC proliferation; on the other hand, in the TME, the secreted pro-AEP was activated to form AEP after a multi-step activation, thus playing its role in extracellular matrix remodeling and the mediation of tumor metastasis (Figure 2B).
AEP inhibitor (AEPI) could effectively impede tumor volume increase and prolong the survival time of mice, thus confirming the feasibility of AEP as a therapeutic target in BC. Moreover, this report provides indirect evidence that AEP may promote tumor progression by regulating the activation of the AKT/PI3K pathway (Figure 2B).
As a family of zinc-dependent endopeptidases, the central role of matrix metalloproteinases (MMPs) in the TME is the degradation and remodeling of the extracellular matrix (ECM). Of the MMP family, MMP-2 and MMP-9 acquire enzymatic activity through the cleavage of AEP. Activated AEP specifically cleaves the asparaginyl bond at the N-terminal of pro-gelatinases, producing a mature form of MMP-2 and MMP-9. Both of them are involved in the degradation of the ECM, which promotes the escape of BC cells in situ and the formation of a metastasis niche [6][7] (Figure 2B).
Tumor-derived AEP harbors an RGD motif that can interact with endothelial integrin αvβ3. The closure of integrin αvβ3 indirectly down-regulates ZO-1 expression via the STAT3 signaling pathway, which finally increases the endothelial permeability in order to assist with the escape of the dissociated tumor (Figure 2B).
Overexpression of AEP is also a high-risk factor for the development of breast ductal carcinoma in situ (DCIS), but the underlying mechanisms promoting the transition from DCIS to invasive disease remain mysterious. The proteolytic properties of AEP and stromal degradation may be the answer to the riddle of DCIS. More evidence is needed to prove this hypothesis [8].
4. Epithelial Ovarian Carcinoma
AEP May Mediate Peritoneal Metastasis in Epithelial Ovarian Carcinoma. Similar to other carcinomas, AEP functionally enhances EOC progression in vitro and in vivo [9]. However, differently from other carcinoma types, AEP is highly expressed in both EOC and human peritoneal mesothelial cells (HPMCs). Integrin α5β1, which is specifically expressed in the EOC, is also highly expressed in both EOC and HPMCs; at the same time, integrin α5β1 and AEP are co-localized in both EOC and HPMCs.
There is great potential for AEP to serve as an effective target in EOC due to its significant roles. The effect of AEPI’s targeting of AEP has been demonstrated in mice. Li X. et al. reported that AEP inhibitor can largely decrease the number of metastases in EOC models, which indicates that there is a great scope for developing therapeutic strategies.
5. Gastric Carcinoma
5.1. Modulation of AEP in GC
Zhang Y. et al. reported that the hypothesis that not can only AEP serve as a therapeutic target and biomarker, but miR-3978 may also enable prediction of peritoneum metastasis as a biomarker in GC (Figure 2D).
5.2. AEP May Promote GC Progression through Diverse Pathways
Previous reports showed that with highly expressed AEP, GC presents a feature of greater invasion and metastasis [10][11]. Cui Y. et al. reported a possible mechanism of AEP’s promotion of GC progression by modulating epithelial-to-mesenchymal transition (EMT). This conclusion was drawn by detecting changes in downstream signaling molecules after knocking down the AEP gene in GC cells. If AEP was knocked down, the expression level of the twist decreased significantly; the epithelial markers’ expression of the EMT and E-cadherin increased, but that of the mesenchymal markers, N-cadherin, β-catenin, and Vimentin decreased. In addition, if AEP was knocked down, the phosphorylation levels of the AKT and MAPK signaling pathways were significantly decreased, which indicated that the AKT and MAPK signaling pathways may be involved in the modulation. More in-depth and detailed studies are needed in order to explain the functional role of AEP in GC (Figure 2D).
5.3. AEP May Play a Crucial Role in Tumor-Associated Macrophages of GC
In the TME of GC, tumor-associated macrophages (TAMs) are vital factors that are presented at different stages of GC progression. TAMs may enhance vascular endothelial growth factor (VEGF) expression to promote angiogenesis and lymph-angiogenesis in GC [12]. In other aspects, TAMs confer cisplatin resistance to GC cells by activating the PI3K/AKT signaling pathway and down-regulating PTEN expression [13].
As a potential biomarker, AEP in GC is not limited in its use for predicting prognosis, but it enables the prediction of tumor metastasis at an early stage. This will greatly improve the efficacy of surgical treatment by intervening in tumor metastasis early, thus eventually improving the post-operative survival of clinical GC patients.
References
- Csoma, C.; Polgar, L. Proteinase from germinating bean cotyledons. Evidence for involvement of a thiol group in catalysis. Biochem. J. 1984, 222, 769–776.
- Chen, J.M.; Dando, P.M.; Rawlings, N.D.; Brown, M.A.; Young, N.E.; Stevens, R.A.; Hewitt, E.; Watts, C.; Barrett, A.J. Cloning, isolation, and characterization of mammalian legumain, an asparaginyl endopeptidase. J. Biol. Chem. 1997, 272, 8090–8098.
- Lin, Y.; Liao, K.; Miao, Y.; Qian, Z.; Fang, Z.; Yang, X.; Nie, Q.; Jiang, G.; Liu, J.; Yu, Y.; et al. Role of Asparagine Endopeptidase in Mediating Wild-Type p53 Inactivation of Glioblastoma. J. Natl. Cancer Inst. 2020, 112, 343–355.
- Lin, Y.; Qiu, Y.; Xu, C.; Liu, Q.; Peng, B.; Kaufmann, G.F.; Chen, X.; Lan, B.; Wei, C.; Lu, D.; et al. Functional role of asparaginyl endopeptidase ubiquitination by TRAF6 in tumor invasion and metastasis. J. Natl. Cancer Inst. 2014, 106, dju012.
- Wang, X.; Song, X.; Zhuo, W.; Fu, Y.; Shi, H.; Liang, Y.; Tong, M.; Chang, G.; Luo, Y. The regulatory mechanism of Hsp90alpha secretion and its function in tumor malignancy. Proc. Natl. Acad. Sci. USA 2009, 106, 21288–21293.
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67.
- Chen, J.M.; Fortunato, M.; Stevens, R.A.; Barrett, A.J. Activation of progelatinase A by mammalian legumain, a recently discovered cysteine proteinase. Biol. Chem. 2001, 382, 777–783.
- Toss, M.S.; Miligy, I.M.; Gorringe, K.L.; McCaffrey, L.; AlKawaz, A.; Abidi, A.; Ellis, I.O.; Green, A.R.; Rakha, E.A. Legumain is an independent predictor for invasive recurrence in breast ductal carcinoma in situ. Mod. Pathol. 2019, 32, 639–649.
- Zhu, Q.; Tang, M.; Wang, X. The expression of asparaginyl endopeptidase promotes growth potential in epithelial ovarian cancer. Cancer Biol. Ther. 2017, 18, 222–228.
- Wang, Y.; Zhang, S.; Wang, H.; Cui, Y.; Wang, Z.; Cheng, X.; Li, W.; Hou, J.; Ji, Y.; Liu, T. High Level of Legumain Was Correlated With Worse Prognosis and Peritoneal Metastasis in Gastric Cancer Patients. Front. Oncol. 2020, 10, 966.
- Li, N.; Liu, Q.; Su, Q.; Wei, C.; Lan, B.; Wang, J.; Bao, G.; Yan, F.; Yu, Y.; Peng, B.; et al. Effects of legumain as a potential prognostic factor on gastric cancers. Med. Oncol. 2013, 30, 621.
- Wu, H.; Xu, J.B.; He, Y.L.; Peng, J.J.; Zhang, X.H.; Chen, C.Q.; Li, W.; Cai, S.R. Tumor-associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J. Surg. Oncol. 2012, 106, 462–468.
- Zheng, P.; Chen, L.; Yuan, X.; Luo, Q.; Liu, Y.; Xie, G.; Ma, Y.; Shen, L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 53.




