
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Osama Al-Madanat | + 1872 word(s) | 1872 | 2021-04-22 04:07:21 | | | |
| 2 | Catherine Yang | Meta information modification | 1872 | 2021-05-20 04:02:44 | | |
Video Upload Options
In recent years, the intensification of human activities including rapid urbanization, industrialization, population, and economic growth, led to an increase in waste production and energy demand. Most importantly such activities pose concerns for health, energy security and climate changes. Hazardous volatile organic compounds, VOC, and aromatic organic compounds, AOC, are being generated from the activities of many vital industries like mining and petrochemicals. They are instrumental in the economic growth of many countries and their products are regarded as privileges to modern communities. Nevertheless, they are toxic and carcinogenic thus, these wastes have been classified as “hazardous”. The simultaneous treatment of organic pollutants and energy recovery is an attractive solution to reduce pollution in water, air, and soil as well as provide alternative clean energy sources. Hydrogen could be generated from organic pollutants in water through photocatalysis. Photocatalysis refers to the oxidation and reduction, redox, reactions on semiconductor surfaces, mediated by the valence band holes and conduction band electrons, which are generated by the absorption of ultraviolet or visible light radiation. Compared to traditional oxidation processes, photocatalytic redox reaction operates at ambient conditions without a high temperature or high pressure, and many recalcitrant organic contaminants can be degraded without the addition of chemical oxidants, hence it is fully green process. Among the various photocatalysts, TiO2, as the most widely employed “golden” photocatalyst, has been largely used in photocatalysis, due to its chemical stability, nontoxicity, and low cost. In the last two decades, TiO2 photocatalysis has expanded very quickly, having undergone various development‐related energy issues and environmental issues, such as direct solar H2O splitting into H2 and the decomposition of pollutants in air and H2O at low concentrations. Although great progress has been made in TiO2 photocatalysis, much remains unknown, which raises an interesting challenge not only for engineers but also for basic scientists. a typical photocatalytic reaction in TiO2 photocatalysis contains many fundamental processes, including charge carrier formation, separation, relaxation, trapping, transfer, recombination, and transportation.
1. Introduction
Water is essential for the existence of all living beings. However, its pollution with organic and inorganic compounds remains a threat and poses great risks to the environment and human health. The water quality is merely a concept reflecting the kind and quantity of contaminants contained in it. Mining and petrochemical industries are instrumental in the economic growth of many countries and their products are regarded as privileges to modern communities [1]. However, the wastes generated from the activities of these industries are toxic and carcinogenic [2]. Thus, these wastes have been classified as “hazardous” [3], and there is a constant increase in the pollution concerns associated with various petrochemical compounds and their by-products in the form of water, air, and soil pollution. Many of these by-products are still extensively employed, especially in the chemical, medical, and other industrial fields, as irreplaceable and inevitable raw materials [4][5]. Aromatic compounds, such as benzene, phenol, and chlorobenzene, are some of the most encountered volatile organic compounds (VOCs). The primary sources of VOCs are originated from a large number of anthropogenic activities, such as refinery streams, especially from catalytic reforming and cracking, and petroleum refining, petrochemical processing, and solvent use [6]. Other VOCs, such as methane and chlorofluorocarbons, are classified as “greenhouse gases”, which cause global warming.
The aromatic ring is the basic constituent of many organic pollutants, such as polyaromatic hydrocarbons (PAHs), dyes, pesticides, and pharmaceuticals. Aromatic compounds, such as benzene, phenols, and benzoic acid, are the most frequently used model substrates to investigate the photocatalytic mechanism and to test the activity of the photocatalysts [7]]. Detailed studies have been made on the harm caused by the aromatic compounds, for example, the potential relationship between the benzene-related compounds and the risk of hematologic cancers, such as lymphoid malignancies [8]. Moreover, long-term exposure to a low concentration of such compounds could predispose to the development of type 2 diabetes (T2D) and affect human metabolism [9]. Aromatic organic compounds also contribute to serious environmental problems, such as water pollution, which may result in the demise of scarce species, and biological genetic variation, which in many cases is an irreversible problem [10].
2. Aromatic Hydrocarbons as Water Pollutants
2.1. Phenols
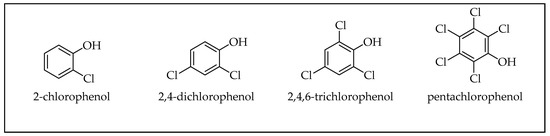
2.2. Polyaromatic Hydrocarbons
| Compound Name | Chemical Structure | Chemical Formula |
Number of Rings | Molecular Weight (g\mol) |
Melting Point (°C) |
Boiling Point (°C) |
Aqueous Solubility (mg/L) |
Vapor Pressure (Pa) |
Log Kow |
|---|---|---|---|---|---|---|---|---|---|
| Naphthalene | 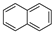 |
C10H8 | 2 | 128.17 | 80.26 | 218 | 31 | 1.0 × 102 | 3.37 |
| Acenaphthene | 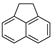 |
C12H10 | 3 | 154.21 | 93.4 | 279 | 3.8 | 3.0 × 10−1 | 3.92 |
| Acenaphthylene | 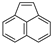 |
C12H8 | 3 | 152.19 | 92–93 | 265–275 | 16 | 9.0 × 10−1 | 4.00 |
| Fluorene | 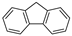 |
C13H10 | 3 | 166.22 | 116–117 | 295 | 1.9 | 9.0 × 10−2 | 4.18 |
| Anthracene | 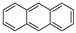 |
C14H10 | 3 | 178.23 | 218 | 340–342 | 0.045 | 1.0 × 10−3 | 4.54 |
| Phenanthrene | 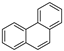 |
C14H10 | 3 | 178.23 | 100 | 340 | 1.1 | 2.0 × 10−2 | 4.57 |
| Fluoranthene | 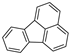 |
C16H10 | 4 | 202.25 | 110.8 | 375 | 0.26 | 1.2 × 10−3 | 5.22 |
| Pyrene | 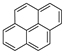 |
C16H10 | 4 | 202.25 | 156 | 393–404 | 0.13 | 6.0 × 10−4 | 5.18 |
| Benzo[a]anthracene | 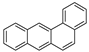 |
C20H12 | 4 | 228.29 | 158 | 438 | 0.011 | 2.8 × 10−5 | 5.91 |
| Chrysene | 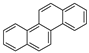 |
C18H12 | 4 | 228.29 | 254 | 448 | 0.006 | 5.7 × 10−7 | 5.91 |
| Benzo[b]fluoranthene | 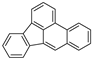 |
C20H12 | 5 | 252.31 | 168.3 | No data | 0.0015 | - | 5.80 |
| Benzo[k]fluoranthene | 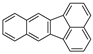 |
C20H12 | 5 | 252.31 | 215.7 | 480 | 0.0008 | 5.2 × 10−8 | 6.00 |
| Benzo[a]pyrene | 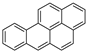 |
C20H12 | 5 | 252.31 | 179–179.3 | 495 | 0.0038 | 7.0 × 10−7 | 5.91 |
| Dibenzo[a,h]anthracene | 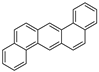 |
C22H14 | 6 | 278.35 | 262 | No data | 0.0006 | 3.7 × 10−10 | 6.75 |
| Benzo[ghi]perylene | 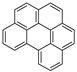 |
C22H12 | 6 | 276.33 | 273 | 550 | 0.00026 | 1.4 × 10−8 | 6.50 |
| Indeno[1,2,3-cd]pyrene | 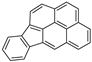 |
C22H12 | 6 | 276.33 | 163.6 | 530 | 0.00019 | - | 6.50 |
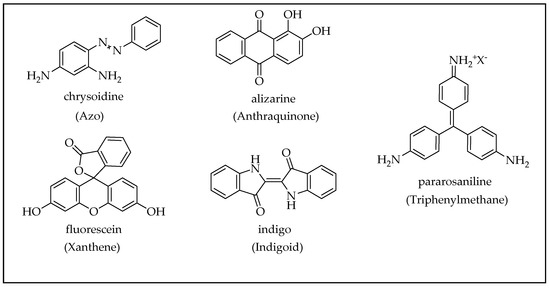
2.3. Organic Dyes
References
- Liew, W.T.; Adhitya, A.; Srinivasan, R. Sustainability trends in the process industries: A text mining-based analysis. Comput. Ind. 2014, 65, 393–400.
- Mechhoud, E.; Rouainia, M.; Rodriguez, M. A new tool for risk analysis and assessment in petrochemical plants. Alex. Eng. J. 2016, 55, 2919–2931.
- Rovira, E.; Cuadras, A.; Aguilar, X.; Esteban, L.; Borràs-Santos, A.; Zock, J.-P.; Sunyer, J. Asthma, respiratory symptoms and lung function in children living near a petrochemical site. Environ. Res. 2014, 133, 156–163.
- Villanueva, F.; Tapia, A.; Lara, S.; Amo-Salas, M. Indoor and outdoor air concentrations of volatile organic compounds and NO2 in schools of urban, industrial and rural areas in Central-Southern Spain. Sci. Total Environ. 2018, 622–623, 222–235.
- Bari, M.A.; Kindzierski, W.B. Ambient volatile organic compounds (VOCs) in communities of the Athabasca oil sands region: Sources and screening health risk assessment. Environ. Pollut. 2018, 235, 602–614.
- Grigoryan, H.; Edmands, W.M.B.; Lan, Q.; Carlsson, H.; Vermeulen, R.; Zhang, L.; Yin, S.N.; Li, G.L.; Smith, M.T.; Rothman, N.; et al. Adductomic signatures of benzene exposure provide insights into cancer induction. Carcinogenesis 2018, 39, 661–668.
- Alegría, M.; Aliaga, J.; Ballesteros, L.; Sotomayor-Torres, C.; González, G.; Benavente, E. Layered nanocomposite 2D-TiO2 with Cu2O nanoparticles as an efficient photocatalyst for 4-Chlorophenol degradation and hydrogen evolution. Top. Catal. 2020.
- Teras, L.R.; Diver, W.R.; Deubler, E.L.; Krewski, D.; Flowers, C.R.; Switchenko, J.M.; Gapstur, S.M. Residential ambient benzene exposure in the United States and subsequent risk of hematologic malignancies. Int. J. Cancer 2019, 145, 2647–2660.
- Mustieles, V.; Fernández, M.F.; Martin-Olmedo, P.; González-Alzaga, B.; Fontalba-Navas, A.; Hauser, R.; Olea, N.; Arrebola, J.P. Human adipose tissue levels of persistent organic pollutants and metabolic syndrome components: Combining a cross-sectional with a 10-year longitudinal study using a multi-pollutant approach. Environ. Int. 2017, 104, 48–57.
- Liu, L.; Li, J.; Zhang, H.; Li, L.; Zhou, P.; Meng, X.; Guo, M.; Jia, J.; Sun, T. In situ fabrication of highly active γ-MnO2/SmMnO3 catalyst for deep catalytic oxidation of gaseous benzene, ethylbenzene, toluene, and o-xylene. J. Hazard. Mater. 2019, 362, 178–186.
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Azab, M.S. An eco-friendly approach to textile and tannery wastewater treatment using maghemite nanoparticles (γ-Fe2O3-NPs) fabricated by Penicillium expansum strain (K-w). J. Environ. Chem. Eng. 2021, 9, 104693.
- Angaru, G.K.R.; Choi, Y.L.; Lingamdinne, L.P.; Choi, J.S.; Kim, D.S.; Koduru, J.R.; Yang, J.K.; Chang, Y.Y. Facile synthesis of economical feasible fly ash-based zeolite-supported nano zerovalent iron and nickel bimetallic composite for the potential removal of heavy metals from industrial effluents. Chemosphere 2021, 267, 128889.
- Al-Nasir, F.M.; Jiries, A.G.; Al-Rabadi, G.J.; Alu’datt, M.H.; Tranchant, C.C.; Al-Dalain, S.A.; Alrabadi, N.; Madanat, O.Y.; Al-Dmour, R.S. Determination of pesticide residues in selected citrus fruits and vegetables cultivated in the Jordan Valley. LWT 2020, 123, 109005.
- Lei, M.; Gao, Q.; Zhou, K.; Gogoi, P.; Liu, J.; Wang, J.; Song, H.; Wang, S.; Liu, X. Catalytic degradation and mineralization mechanism of 4-chlorophenol oxidized by phosphomolybdic acid/H2O2. Sep. Purif. Technol. 2021, 257, 117933.
- Al Nasir, F.; Batarseh, M.I. Agricultural reuse of r 456545eclaimed water and uptake of organic compounds: Pilot study at Mutah University wastewater treatment plant, Jordan. Chemosphere 2008, 72, 1203–1214.
- Lin, Z.; Li, L.; Yu, L.; Li, W.; Yang, G. Dual-functional photocatalysis for hydrogen evolution from industrial wastewaters. Phys. Chem. Chem. Phys. 2017, 19, 8356–8362.
- AlSalka, Y.; Karabet, F.; Hashem, S. Development and optimisation of quantitative analytical method to determine BTEX in environmental water samples using HPLC-DAD. Anal. Methods 2010, 2, 1026–1035.
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A short review of techniques for phenol removal from wastewater. Curr. Pollut. Rep. 2016, 2, 157–167.
- Michałowicz, J.; Duda, W. Phenols—Sources and toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362.
- Zango, Z.U.; Sambudi, N.S.; Jumbri, K.; Ramli, A.; Abu Bakar, N.H.H.; Saad, B.; Rozaini, M.N.H.; Isiyaka, H.A.; Osman, A.M.; Sulieman, A. An overview and evaluation of highly porous adsorbent materials for polycyclic aromatic hydrocarbons and phenols removal from wastewater. Water 2020, 12, 2921.
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic Compounds in Water: Sources, Reactivity, Toxicity and Treatment Methods, Phenolic Compounds—Natural Sources, Importance and Applications; IntechOpen-Open Science Open Minds: London, UK, 2017.
- Igbinosa, E.O.; Odjadjare, E.E.; Chigor, V.N.; Igbinosa, I.H.; Emoghene, A.O.; Ekhaise, F.O.; Igiehon, N.O.; Idemudia, O.G. Toxicological profile of chlorophenols and their derivatives in the environment: The public health perspective. Sci. World J. 2013, 2013, 460215.
- Alsalka, Y.; Karabet, F.; Hashem, S. Evaluation of electrochemical processes for the removal of several target aromatic hydrocarbons from petroleum contaminated water. J. Environ. Monit. 2011, 13, 605–613.
- Drwal, E.; Rak, A.; Gregoraszczuk, E.L. Review: Polycyclic aromatic hydrocarbons (PAHs)-Action on placental function and health risks in future life of newborns. Toxicology 2019, 411, 133–142.
- Jiries, A.G.; Hussein, H.H.; Lintelmann, J. Polycyclic aromatic hydrocarbon in rain and street runoff in Amman, Jordan. J. Environ. Sci. 2003, 15, 848–853.
- Huang, H.; Buekens, A. Chlorinated dioxins and furans as trace products of combustion: Some theoretical aspects. Toxicol. Environ. Chem. 2000, 74, 179–193.
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic aromatic hydrocarbons: Sources, toxicity, and remediation approaches. Front. Microbiol. 2020, 11, 562813.
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123.
- Majumdar, D.; Rajaram, B.; Meshram, S.; Suryawanshi, P.; Chalapati Rao, C.V. Worldwide distribution of polyclyclic aromatic hydrocarbons in urban road dust. Int. J. Environ. Sci. Technol. 2016, 14, 397–420.
- Forsgren, A.J. Wastewater Treatment: Occurrence and Fate of Polycyclic Aromatic Hydrocarbons (PAHs), 1st ed.; CRC Press: Boca Raton, FL, USA, 2015.
- Yan, J.; Wang, L.; Fu, P.P.; Yu, H. Photomutagenicity of 16 polycyclic aromatic hydrocarbons from the US EPA priority pollutant list. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2004, 557, 99–108.
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921.
- Jia, C.; Batterman, S. A critical review of naphthalene sources and exposures relevant to indoor and outdoor air. Int. J. Environ. Res. Public. Health 2010, 7, 2903–2939.
- Sudakin, D.L.; Stone, D.L.; Power, L. Naphthalene mothballs: Emerging and recurring issues and their relevance to environmental health. Curr. Top. Toxicol. 2011, 7, 13–19.
- Tian, W.; Bai, J.; Liu, K.; Sun, H.; Zhao, Y. Occurrence and removal of polycyclic aromatic hydrocarbons in the wastewater treatment process. Ecotoxicol. Environ. Saf. 2012, 82, 1–7.
- Pereira, L.; Alves, M. Dyes—Environmental impact and remediation. In Environmental Protection Strategies for Sustainable Development; Malik, A., Grohmann, E., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 111–162.
- Hunger, K.; Gregory, P.; Miederer, P.; Berneth, H.; Heid, C.; Mennicke, W. Important chemical chromophores of dye classes. In Industrial Dyes, Hunger, K., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2002; pp. 13–112.
- Ghodke, S.A.; Sonawane, S.H.; Bhanvase, B.A.; Potoroko, I. Advanced engineered nanomaterials for the treatment of wastewater. In Handbook of Nanomaterials for Industrial Applications; Mustansar Hussain, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 959–970.
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour. Technol. 2003, 87, 129–132.
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971.




