
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jamie O'Reilly | + 1372 word(s) | 1372 | 2021-05-17 08:35:32 | | | |
| 2 | Rita Xu | Meta information modification | 1372 | 2021-05-19 05:26:59 | | |
Video Upload Options
Mismatch negativity (MMN) is a component of the difference waveform derived from passive auditory oddball stimulation.
1. Introduction
Mismatch negativity (MMN) is generally defined as a negative polarity deflection viewed across the 150–250 ms latency range in difference waveforms derived from standard and deviant event-related potential (ERP) responses elicited by passive auditory oddball paradigms [1][2][3]. This waveform feature is considered to be maximal approximately halfway between the center of the forehead and the apex (electrode Fz in the 10–20 system), although, like other ERP components, its morphology changes depending on the reference electrode(s). In passive auditory oddball paradigm experiments, subjects are instructed not to attend to ongoing changes in the auditory environment. Repetitive identical (i.e., standard) stimuli are played at a constant rate with a defined inter-stimulus interval (ISI) until they are unpredictably swapped for a physically different (i.e., deviant) stimulus. This sequence is then repeated multiple times to obtain enough electroencephalogram (EEG) segments to average together to produce corresponding ERP waveforms. Subtraction of the standard ERP from the deviant ERP is performed, and MMN is typically quantified from this difference waveform somewhere within the aforementioned latency range, although precisely where is generally not standardized between studies. While there are many variants on the theme of passive auditory oddball paradigms, the fundamental principle rests on using two or more physically distinct sounds, with the formation of a relatively predictable pattern using one that is subsequently perturbed by another, physically different sound. This aspect of unpredictable change in the stimulus presentation sequence is crucial to popular interpretations of MMN generation, as discussed later.
MMN is reportedly elicited by any perceptible change in the physical properties of a repeated sound [2][4][5]. This deviance can be made with regard to duration, frequency, loudness, source location, pitch transition, synthesized vowel, and presumably any combination thereof. While their predicted and unpredicted contexts are generally believed to be of critical importance, the magnitude of MMN is proportional to the degree of physical difference between standard and deviant stimuli [1]. Furthermore, changes in individual physical properties of sound are considered to produce distinct attributes in the MMN response, which may be characteristic of dissociable underlying mechanisms. For instance, studies that have applied source modelling techniques have identified separate loci of activity associated with different physical deviances [6][7][8][9]. This is particularly relevant for the substantial body of clinical research (discussed in a separate section below) where different types of physical deviance have been used in passive auditory oddball paradigms to elicit MMN in patient groups. It is considered to be possible to elicit MMN from patients who are sleeping [10], anaesthetized [11], or in comatose states [12]. A corresponding mismatch response (MMR) is also thought to exist in many different animal species, including amphibians [13], lower mammals [14], pigeons [15], dolphins [16] and monkeys [17].
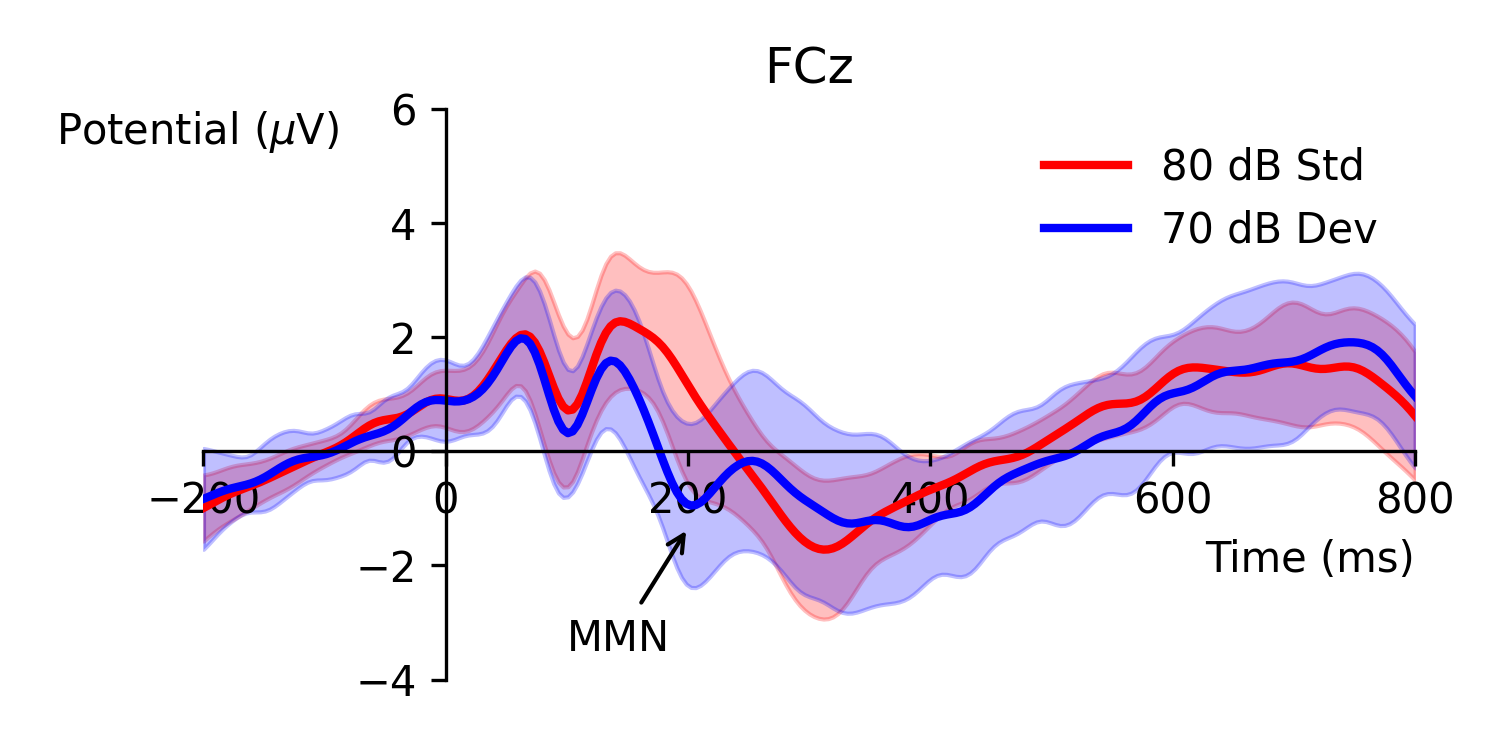 Figure 1. Event-related potential waveforms obtained from an intensity oddball paradigm with quieter deviant (70 dB) and louder standard stimuli (80 dB). Mismatch negativity is typically defined as any observable negative amplitude difference between the two ERPs within the latency range of 150 to 250 ms. The data for this figure was obtained from [1] and includes 40 subjects; shaded error-bars show standard deviation.
Figure 1. Event-related potential waveforms obtained from an intensity oddball paradigm with quieter deviant (70 dB) and louder standard stimuli (80 dB). Mismatch negativity is typically defined as any observable negative amplitude difference between the two ERPs within the latency range of 150 to 250 ms. The data for this figure was obtained from [1] and includes 40 subjects; shaded error-bars show standard deviation.
This concludes the main introductory points concerning MMN without delving into the proposed interpretations of its underlying mechanisms. These may be summarized as follows: (1) it is quantified from the difference wave between standard and deviant ERP responses evoked by a passive auditory oddball paradigm; (2) it can be elicited by any physical difference between standard and deviant stimuli; (3) its magnitude is related to the degree of difference between standard and deviant stimuli; (4) it can be evoked while subjects are awake, asleep, or unconscious; and (5) it is widely shared throughout the animal kingdom. These consistent findings of MMN are themselves evidence of robust sensory processing mechanisms. However, the practical significance of this topic is principally derived from its putative utility as a biomarker for psychiatric disease [18][19][20]. This raises a number of questions concerning its underlying mechanisms that demand greater levels of scrutiny if this promise is to be realized without detriment to prospective patients.
2. Deviance Detection Theory
The mechanisms traditionally believed to underlie MMN generation have recently been expounded in a couple of comprehensive review articles [1][3]. These adequately recapitulate the prevailing theoretical framework, although without sufficiently challenging the limitations of supporting evidence. The present review offers a different perspective in an effort to bring balance to this discussion. From the earliest studies, MMN was interpreted as a difference-detection response that represents automatic stimulus discrimination [21]. This has since been referred to as the deviance detection theory. Using today’s terminology, this theory considers that MMN reflects a neurophysiological prediction error signal elicited by an error in perceptual inference; however, it may be noted that this is essentially a rewording of the initially postulated mechanisms [22]. This account assumes processes of auditory object abstraction, whereby the physical properties of discrete sounds are represented together, which presumably occurs during the ascending pathway as sensory signals travel through brainstem nuclei and up towards the cortex. The means by which this abstraction is formulated may perhaps be through the functions of auditory neurons that are tuned to different physical properties of sounds [23], although the manner in which these cellular activities are integrated is uncertain. Further to the encoding of auditory objects, this theory posits that after being encountered, these auditory objects are stored in sensory memory, or in other words, represented by a perceptual predictive model. It is proposed that this memory representation is automatically compared with incoming auditory objects, and if the two mismatch, the MMN response is generated; thus, it is referred to as a prediction error signal [24]. If the incoming auditory object and expected input are not sufficiently different to initiate an MMN response, repetition suppression or stimulus-specific adaptation (SSA) occurs, which has been referred to as model adjustment [25]. These hypothesized mechanisms related to predictive modelling and comparative processes can be described as top-down, whereas those associated with formation of auditory objects may be referred to as bottom-up. Various terms have been used to describe this theory, including statistical learning and inference, sensory learning, auditory perceptual learning, change detection, sensory memory, predictive coding, auditory pattern learning, prediction error signaling, novelty processing, hierarchical rule learning, and automatic auditory discrimination. Despite the deviance detection theory being widely supported in over four decades of published research [2][21], it is not universally accepted.
3. Fresh Afferents Theory
The term SSA is used to describe a phenomenon observed where repetition of an identical stimulus results in attenuation of its evoked response, or repetition suppression. This is generally accepted as an established property in sensory neurophysiology. Some research [26][27] has examined the deviance detection theory and rejected it by concluding that SSA alone can account for MMN responses. This fresh afferents theory presented an opposition to the deviance detection hypothesis by suggesting that differential SSA to standard and deviant stimuli can sufficiently explain differences between their responses, and corresponding difference waveform defections [26][27]. The fresh afferents theory may be more appealing because it avoids the need to invoke some of the unproven theoretical elements of the deviance detection theory, such as auditory objects, predictive perceptual models and comparative mechanisms. However, this has frequently been portrayed as a less sophisticated means of MMN generation than deviance detection [28][29][30], presumably due to the omission of more elaborate top-down processes. Subsequently SSA was integrated into the deviance detection theory via the model adjustment hypothesis mentioned above [25], which retained the need for deviance-detection mechanisms to generate MMN. Otherwise the fresh afferents theory was thoroughly dismissed by proponents of the deviance detection theory [30]. It may be noted that the emergent definition of a genuine MMN response appears to prohibit any mechanism other than the prevailing deviance-detection hypothesis [31], which may constrain the scope for properly evaluating alternatives. While SSA is repeatedly shown in auditory neurons responding to presentation of tones of different frequency, it is unclear whether comparable processes occur in response to sequences of stimuli with changes in other physical aspects of sound [32]. As such, fresh afferents might not be sufficient to describe findings of MMN in response to changes in different physical parameters, and SSA could perhaps be an over-generalized term that refers predominantly to frequency-specific adaptation, although there have been recent efforts to verify this using frequency-balanced multi-tone stimuli [33].
References
- Fitzgerald, K.; Todd, J. Making Sense of Mismatch Negativity. Front. Psychiatry 2020, 11, 468.
- Näätänen, R.; Kujala, T.; Light, G. Mismatch Negativity: A Window to the Brain; Oxford University Press: Oxford, UK, 2019; ISBN 9780198705079.
- Ross, J.M.; Hamm, J.P. Cortical Microcircuit Mechanisms of Mismatch Negativity and Its Underlying Subcomponents. Front. Neural Circuits 2020, 14, 13.
- Pakarinen, S.; Takegata, R.; Rinne, T.; Huotilainen, M.; Näätänen, R. Measurement of extensive auditory discrimination profiles using the mismatch negativity (MMN) of the auditory event-related potential (ERP). Clin. Neurophysiol. 2007, 118, 177–185.
- Näätänen, R.; Pakarinen, S.; Rinne, T.; Takegata, R. The mismatch negativity (MMN): Towards the optimal paradigm. Clin. Neurophysiol. 2004, 115, 140–144.
- Frodl-Bauch, T.; Kathmann, N.; Möller, H.-J.; Hegerl, U. Dipole Localization and Test-Retest Reliability of Frequency and Duration Mismatch Negativity Generator Processes. Brain Topogr. 1997, 10, 3–8.
- Molholm, S.; Martinez, A.; Ritter, W.; Javitt, D.C.; Foxe, J.J. The Neural Circuitry of Pre-attentive Auditory Change-detection: An fMRI Study of Pitch and Duration Mismatch Negativity generators. Cereb. Cortex 2004, 15, 545–551.
- Rosburg, T. Left hemispheric dipole locations of the neuromagnetic mismatch negativity to frequency, intensity and duration deviants. Cogn. Brain Res. 2003, 16, 83–90.
- An, H.; Auksztulewicz, R.; Kang, H.; Schnupp, J.W. Cortical mapping of mismatch responses to independent acoustic features. Hear. Res. 2020, 399, 107894.
- Loewy, D.H.; Campbell, K.B.; Bastien, C. The mismatch negativity to frequency deviant stimuli during natural sleep. Electroencephalogr. Clin. Neurophysiol. 1996, 98, 493–501.
- Zhang, Y.; Yan, F.; Wang, L.; Wang, Y.; Wang, C.; Wang, Q.; Huang, L. Cortical Areas Associated With Mismatch Negativity: A Connectivity Study Using Propofol Anesthesia. Front. Hum. Neurosci. 2018, 12, 392.
- Fischer, C.; Morlet, D.; Giard, M.-H. Mismatch Negativity and N100 in Comatose Patients. Audiol. Neurotol. 2000, 5, 192–197.
- Yue, X.; Fan, Y.; Xue, F.; Brauth, S.E.; Tang, Y.; Fang, G. The First Call Note Plays a Crucial Role in Frog Vocal Communication. Sci. Rep. 2017, 7, 10128.
- Featherstone, R.E.; Melnychenko, O.; Siegel, S.J. Mismatch negativity in preclinical models of schizophrenia. Schizophr. Res. 2018, 191, 35–42.
- Schall, U.; Müller, B.W.; Kärgel, C.; Güntürkün, O. Electrophysiological mismatch response recorded in awake pigeons from the avian functional equivalent of the primary auditory cortex. NeuroReport 2015, 26, 239–244.
- Romanchek, B.A.; Scheifele, P.M. Investigating Atlantic bottlenose dolphin mismatch negativity response to pure tone stimuli. J. Acoust. Soc. Am. 2019, 145, 1776.
- Fishman, Y.I. The Mechanisms and Meaning of the Mismatch Negativity. Brain Topogr. 2014, 27, 500–526.
- Light, G.A.; Näätänen, R. Mismatch negativity is a breakthrough biomarker for understanding and treating psychotic disorders. Proc. Natl. Acad. Sci. USA 2013, 110, 15175–15176.
- Etodd, J.; Eharms, L.; Eschall, U.; Michie, P.T. Mismatch Negativity: Translating the Potential. Front. Psychiatry 2013, 4, 171.
- Tada, M.; Kirihara, K.; Mizutani, S.; Uka, T.; Kunii, N.; Koshiyama, D.; Fujioka, M.; Usui, K.; Nagai, T.; Araki, T.; et al. Mismatch negativity (MMN) as a tool for translational investigations into early psychosis: A review. Int. J. Psychophysiol. 2019, 145, 5–14.
- Näätänen, R.; Gaillard, A.; Mäntysalo, S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol. 1978, 42, 313–329.
- Näätänen, R.; Paavilainen, P.; Rinne, T.; Alho, K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clin. Neurophysiol. 2007, 118, 2544–2590.
- Rauschecker, J.P.; Romanski, L.M. Auditory Cortical Organization: Evidence for Functional Streams. In The Auditory Cortex; Springer: Boston, MA, USA, 2010; pp. 99–116.
- Wacongne, C.; Changeux, J.-P.; Dehaene, S. A Neuronal Model of Predictive Coding Accounting for the Mismatch Negativity. J. Neurosci. 2012, 32, 3665–3678.
- Garrido, M.I.; Kilner, J.M.; Stephan, K.E.; Friston, K.J. The mismatch negativity: A review of underlying mechanisms. Clin. Neurophysiol. 2009, 120, 453–463.
- Jaaskelainen, I.P.; Ahveninen, J.; Bonmassar, G.; Dale, A.M.; Ilmoniemi, R.J.; Levanen, S.; Lin, F.-H.; May, P.; Melcher, J.; Stufflebeam, S.; et al. Human posterior auditory cortex gates novel sounds to consciousness. Proc. Natl. Acad. Sci. USA 2004, 101, 6809–6814.
- May, P.J.C.; Tiitinen, H. Mismatch negativity (MMN), the deviance-elicited auditory deflection, explained. Psychophysiology 2010, 47, 66–122.
- Kurkela, J.L.O.; Lipponen, A.; Kyläheiko, I.; Astikainen, P. Electrophysiological evidence of memory-based detection of auditory regularity violations in anesthetized mice. Sci. Rep. 2018, 8, 3027.
- Näätänen, R.; Kujala, T.; Escera, C.; Baldeweg, T.; Kreegipuu, K.; Carlson, S.; Ponton, C. The mismatch negativity (MMN)—A unique window to disturbed central auditory processing in ageing and different clinical conditions. Clin. Neurophysiol. 2012, 123, 424–458.
- Naatanen, R.; Jacobsen, T.; Winkler, I. Memory-based or afferent processes in mismatch negativity (MMN): A review of the evidence. Psychophysiology 2005, 42, 25–32.
- Harms, L.; Michie, P.T.; Näätänen, R. Criteria for determining whether mismatch responses exist in animal models: Focus on rodents. Biol. Psychol. 2016, 116, 28–35.
- Duque, D.; Wang, X.; Nieto-Diego, J.; Krumbholz, K.; Malmierca, M.S. Neurons in the inferior colliculus of the rat show stimulus-specific adaptation for frequency, but not for intensity. Sci. Rep. 2016, 6, 24114.
- Harpaz, M.; Jankowski, M.M.; Khouri, L.; Nelken, I. Emergence of abstract sound representations in the ascending auditory system. Prog. Neurobiol. 2021, 102049.





