
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio Palumbo Jr | + 1564 word(s) | 1564 | 2021-04-27 10:40:30 | | | |
| 2 | Vicky Zhou | Meta information modification | 1564 | 2021-05-19 04:11:51 | | |
Video Upload Options
Head and neck squamous cell carcinomas (HNSCC) are among the most common and lethal tumors worldwide, occurring mostly in oral cavity, pharynx, and larynx tissues. The squamous epithelia homeostasis is supported by the extracellular matrix (ECM), and alterations in this compartment are crucial for cancer development and progression. Laminin is a fundamental component of ECM, where it represents one of the main components of basement membrane (BM), and data supporting its contribution to HNSCC genesis and progression has been vastly explored in oral cavity squamous cell carcinoma. Laminin subtypes 111 (LN-111) and 332 (LN-332) are the main isoforms associated with malignant transformation, contributing to proliferation, adhesion, migration, invasion, and metastasis, due to its involvement in the regulation of several pathways associated with HNSCC carcinogenesis, including the activation of the EGFR/MAPK signaling pathway. Therefore, it draws attention to the possibility that laminin may represent a convergence point in HNSCC natural history, and an attractive potential therapeutic target for these tumors.
1. Introduction
Head and neck cancer (HNC) are among the most common and lethal tumors worldwide, affecting mostly men and populations from low- and middle-income countries [1][2]. The great majority of HNC originate from the squamous cells lining the mucosal epithelium in head and neck sites, being collectively named head and neck squamous cell carcinomas (HNSCC). The head and neck anatomical sites more frequently affected by the development of HNSCC are the oral cavity, pharynx, and larynx [3][4]. Together, these three anatomical sites congregate the vast majority of HNSCC cases and the major number of deaths, as previously estimated [1][5] and summarized in Table 1.
Table 1. Estimated numbers of new cases and deaths per year worldwide for the most common types of head and neck squamous cell carcinomas.
| Type of Head and Neck Squamous Cell Carcinoma | New Cases per Year Worldwide [1][5] | Deaths per Year Worldwide [1][5] |
|---|---|---|
| Oral Cavity Squamous Cell Carcinoma | 355,000/male-to-female incidence ratio of 2:1 | 177,000 |
| Pharyngeal Squamous Cell Carcinoma | 302,000 | 159,000 |
| Laryngeal Squamous Cell Carcinoma | 177,000/male-to-female incidence ratio of 7:1 | 95,000 |
The main risk factors associated with these tumors are tobacco smoking and alcohol consumption [6]. Additionally, viral infections caused by Human Papilloma Virus (HPV) and Epstein Barr Virus (EBV) are highly associated with oropharynx and nasopharynx development, respectively [6].
HNSCC presents poor prognosis since most patients are diagnosed presenting advanced-stage tumors [7]. HNSCC treatment protocols can include surgery, radiotherapy, chemotherapy, targeted therapy, or a combination of treatments, depending on the location and stage of the tumor, patient age, and health condition [8]. The high incidence and lethality of these tumors, together with their poor prognosis, draw attention to need for deeper understanding their biology to identify potential biomarkers and therapeutic strategies to improve patient management and survival.
Molecular characterization of HNSCC has shown a great heterogeneity in molecular alterations present in these tumors, not only because of the different sites analyzed together, but also because of the low frequency of specific genetic alterations, even when a specific site is considered [9]. This characteristic hampers the development of target therapies based on the genetic alterations present in the neoplastic cells. However, numerous studies have shown the fundamental role of the extracellular matrix (ECM) during cancer development and progression, as well as its potential as therapeutic targets [10][11][12]. In fact, the relevance of ECM to cancer progression and treatment has long been established and is originated from the observation that an increase in ECM deposition occurs in the evolution of malignant neoplasms and is associated with poor patient prognosis and treatment resistance [13].
ECM includes the interstitial matrix and the basement membrane (BM) [14]. Although the interstitial matrix is composed of polysaccharides and fibrous proteins that fill the spaces between cells and acts as a sort of buffer against mechanical stresses and strains placed on the ECM [14], BM are sheet-like specialized ECM regions that surround most animal tissues [15]. BM functions are quite diverse, not only involving physical roles such as anchoring the epithelium, but also maintaining tissue integrity and acting by storing growth factors and cytokines, functioning as a bridge between physical forces and biochemical signaling [15]. The main components of BM are laminins, collagen IV, nidogens and the proteoglycans perlecan and agrine [15]. Defects in BM assembly or composition result in a multitude of human diseases. Moreover, although traditionally viewed as a protective structure to defend tissues against cancer spread and invasion, BM dysregulation is a hallmark of many cancers [16]. BM degradation by proteases, specifically matrix metalloproteinases (MMP -1, -3, -7, -9, -10, -12, -13 and -19) produced by tumor cells, such as carcinoma, is also followed by the production of their own array of molecules, used as substrates for cancer cell invasion and proliferation. Although tumor-derived BM molecules do not form a similar network as those from normal tissues, there are striking intramolecular interactions followed by dynamic modification occurring in the newly formed BM that are crucial in supporting cancer development [16]. In this sense, laminins are glycoproteins of high molecular weight (approximately 400 to 900 KDa) found in the BM of several epithelial tissues and are presented in the shape of a cross or T, formed by three interlaced chains called α, β and γ. They were first identified in Engelbreth–Holm–Swarm sarcoma, a tumor that produces large amounts of basement membrane [17]. Laminins are known to directly regulate crucial biological events associated with morphogenesis, such as proliferation, adhesion, migration, angiogenesis, and survival. However, alterations in laminin expression pattern as well as activity are associated with pathological events, for instance the carcinogenic process, since they can modulate key cellular processes thus influencing different cellular behaviors [18][19], thus increasing their relevance as a prominent therapeutic target [18]. In addition, several studies have shown that the alterations in laminin expression pattern and activity in tumor tissues are associated with patient outcomes, such as tumor invasiveness and poor prognosis, revealing its potential as prognosis biomarker [20][21].
2. Laminin as a Therapeutic Target for HNSCC—Future Perspectives
Laminin has clear contribution for the progression of HNSCC, particularly for those of oral cavity, pharynx, and larynx. In this sense, the aberrant expression of LN332 seems to represent the main characteristics which connect the distinct head and neck squamous tumors, since several studies have been shown that the LN332, and particularly its γ2 chain overexpression, are associated with tumor progression of oral cavity, pharynx, and larynx tissues. However, in the development of OCSCC, despite the relevance of LN332/γ2 chain expression, the biological fragments which arise from the degradation not only from LN332 but also from LN111 may play a remarkable role in the carcinogenesis of oral tissue. In fact, it was previously reported that the laminin fragments activate EGFR/MAPK as well as PI3K/AKT signaling pathways, consequently triggering crucial cellular mechanisms, such as EMT, migration, and invasion (Figure 1). Although the scarce literature demonstrates that laminin is involved with the progression of pharyngeal and laryngeal squamous cell carcinoma tumors towards a more aggressive and invasive phenotype, the mechanisms underlying this phenomenon are not yet reported, mainly because of limited in vitro and in vivo models currently available for the study of these tumors. Nevertheless, based on tumor similarities, one could suggest that similar signaling pathways may be modulated in pharyngeal and laryngeal squamous cell carcinomas, representing common mechanisms by which laminin plays a role in HNSCC progression. Therefore, the data discussed here draws attention to the possibility that laminin may represent a convergence point in HNSCC natural history, and an attractive potential therapeutic target for these tumors (Figure 2).
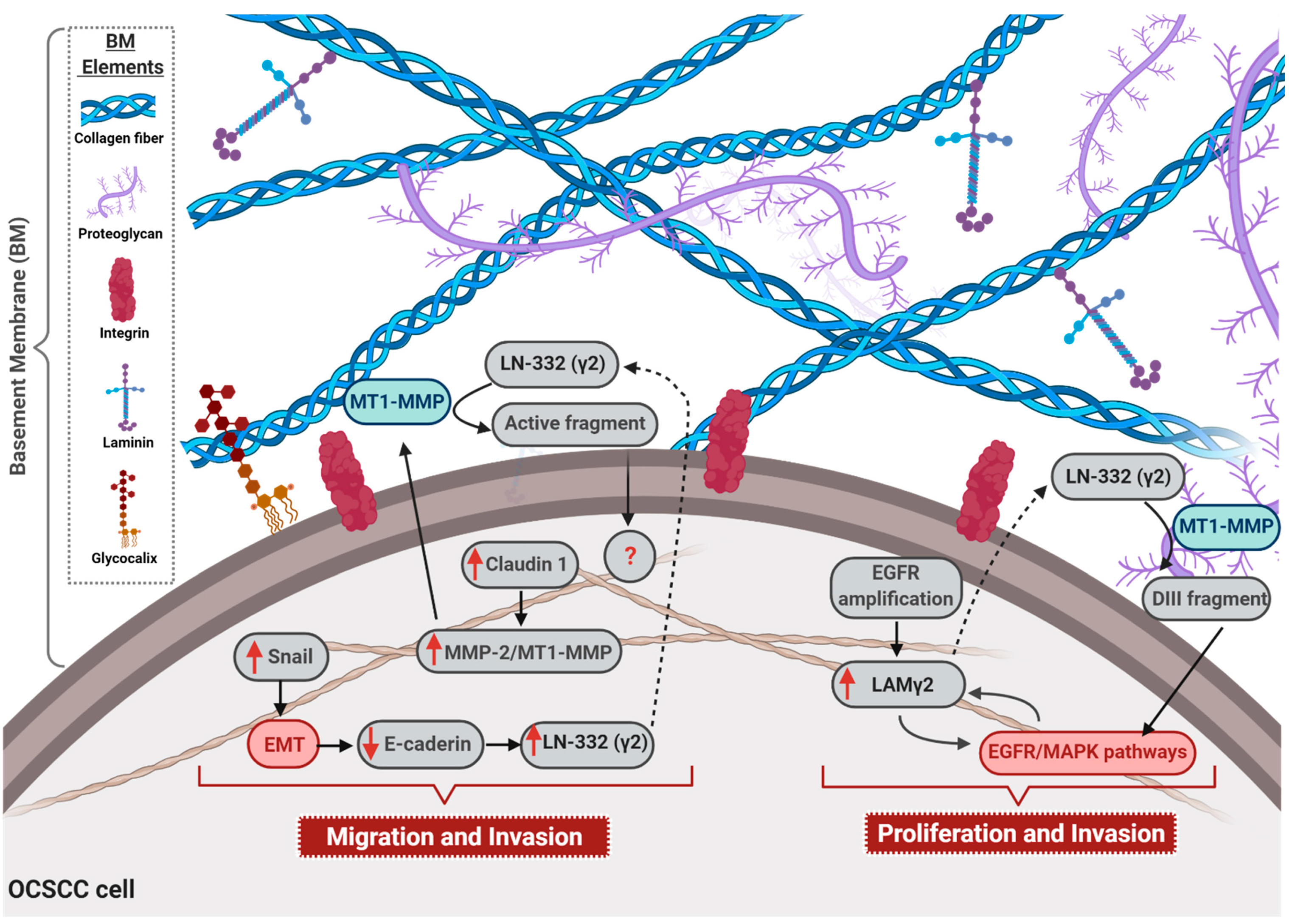
Figure 1. Role of laminin in oral cavity squamous cell carcinomas (OCSCC) progression. The figure illustrates the main mechanisms through which laminin is involved in OCSCC malignant phenotype acquisition. Up-regulation of the transcription factor Snail activates epithelial–mesenchymal transition (EMT), and consequently e-cadherin down-regulation, which enhances laminin 332 (LN-332) γ2 chain expression. Additionally, overexpression of claudin-1 leads to an increase in the expression of matrix metalloproteinase 2 (MMP-2) and membrane type 1 (MT1)—MMP, as well as to the cleavage rate of LN-332 (γ2) by these proteases, culminating in the migration and invasion of OCSCC. Also, EGFR gene amplification is associated with overexpression of γ2 chain (LAM γ2) that, in turn, hyperactivates EGFR/MAPK signaling pathway and, in a feedback circuit, increases LAM γ2expression. Moreover, the secreted LN-332 (γ2) is cleaved by MMP2 and MT1-MMP, generating the DIII fragment that can bind to EGFR, ending up in the activation of EGFR/MAPK pathway. Finally, the activation of EGFR/MAPK signaling pathway enhances proliferation and invasion rates of OCSCC cells. Dotted arrows: molecules secreted by OCSCC cells; solid arrows: activation of cellular events or intracellular signaling pathways; red solid arrow: overexpressed molecules; red boxes: activated signaling pathways; blue boxes: cleavage by MT1-MMP. BM = basement membrane.
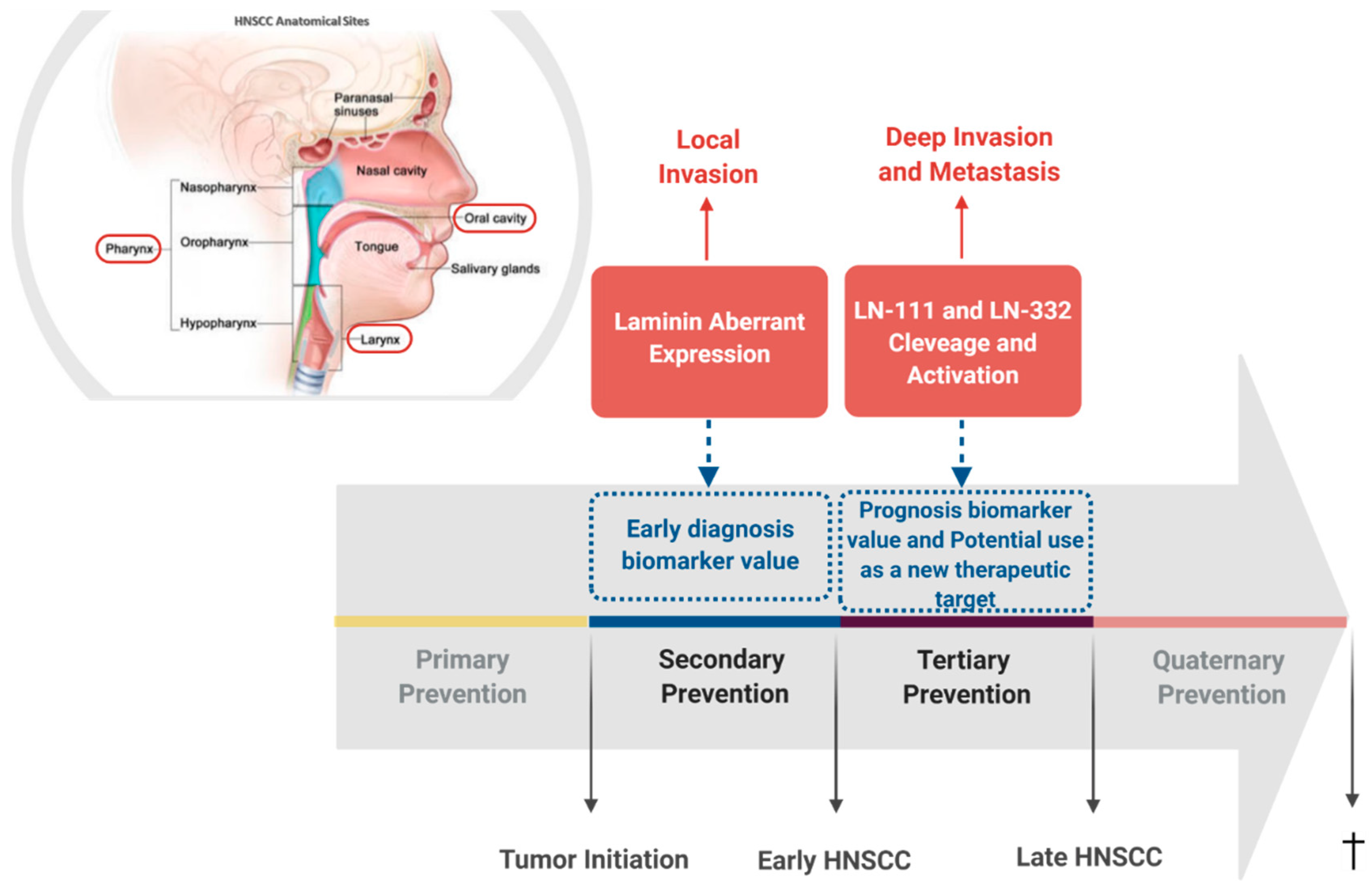
Figure 2. Laminin involvement in head and neck squamous cell carcinomas (HNSCC) progression. Schematic representation of HNSCC most frequent affected anatomical sites and summary of the already identified involvement of laminin in HNSCC, pointing out its intervention potential in tumor’s natural history. Head and neck anatomical sites from where the squamous cell carcinomas discussed in this review arise are highlighted by a red frame. Secondary prevention is highlighted since the aberrant expression pattern of laminin in HNSCC unveils a potential value of its use as early diagnosis biomarker. Likewise, tertiary prevention is also highlighted since cleavage and activation of laminin trigger intracellular signaling pathways that lead to the acquisition of a malignant phenotype and may represent a potential new therapeutic target. Additionally, cleavage and activation of laminin are associated with patient outcome, indicating a potential value of its use as prognosis biomarker.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Anantharaman, D.; Brennan, P.; Leemans, C.R. Head and Neck Cancers: New Etiological Insights. In World Cancer Report 2020, 1st ed.; Wild, C.P., Weiderpass, E., Stewart, B.W., Eds.; International Agency for Research on Cancer: Lyon, France, 2020; Volume 1, pp. 310–322.
- National Cancer Institute. Head and Neck Cancers. Available online: (accessed on 12 September 2020).
- Mishra, M.S.; Upadhyaya, N.; Dive, A.M.; Bodhade, A.S. Histological patterns of head and neck tumors: An insight to tumor histology. J. Oral Maxillofac. Pathol. 2014, 18, 58–68.
- Bradford, C.R.; Ferlito, A.; Devaney, K.O.; Mäkitie, A.A.; Rinaldo, A. Prognostic factors in laryngeal squamous cell carcinoma. Laryngoscope Investig. Otolaryngol. 2020, 5, 74–81.
- Pezzuto, F.; Buonaguro, L.; Caponigro, F.; Ionna, F.; Starita, N.; Annunziata, C.; Buonaguro, F.M.; Tornesello, M.L. Update on Head and Neck Cancer: Current Knowledge on Epidemiology, Risk Factors, Molecular Features and Novel Therapies. Oncology 2015, 89, 125–136.
- Pfister, D.G.; Spencer, S.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; Colevas, A.D.; Dunphy, F.; Eisele, D.W. Head and neck cancers, version 2. 2014: Clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2014, 12, 1454–1487.
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72.
- The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582.
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014, 15, 1243–1253.
- Walker, C.; Mojares, E.; Hernández, A.D.R. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028.
- Palumbo, A., Jr.; Meireles Da Costa, N.; Pontes, B.; Oliveira, F.L.; Codeço, M.L.; Ribeiro Pinto, L.F.; Nasciutti, L.E. Esophageal Cancer Development: Crucial Clues Arising from the Extracellular Matrix. Cells 2020, 9, 455–465.
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160.
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27.
- Jayadev, R.; Sherwood, D.R. Basement membranes. Curr. Biol. 2017, 27, R207–R211.
- Liotta, L.A.; Rao, C.N.; Wewer, U.M. Biochemical interactions of tumor cells with the basement membrane. Annu. Rev. Biochem. 1986, 55, 1037–1057.
- Timpl, R.; Rohde, H.; Robey, P.G.; Rennard, S.I.; Foidart, J.M.; Martin, G.R. Laminin--a glycoprotein from basement membranes. J. Biol. Chem. 1979, 254, 9933–9937.
- Jourquin, J.; Tripathi, M.; Guess, C.; Quaranta, V. Laminins and Cancer Progression. In Cell-Extracellular Matrix Interactions in Cancer; Zent, R., Pozzi, A., Eds.; Springer: New York, NY, USA, 2009; Volume 1, pp. 87–109.
- Ramovs, V.; Molder, L.T.; Sonnenberg, A. The opposing roles of laminin-binding integrins in cancer. Matrix Biol. 2017, 57–58, 213–243.
- Garg, M.; Kanojia, D.; Okamoto, R.; Jain, S.; Madan, V.; Chien, W.; Sampath, A.; Ding, L.-W.; Xuan, M.; Said, J.W.; et al. Laminin-5 gamma-2 (LAMC2) is highly expressed in anaplastic thyroid carcinoma and is associated with tumor progression, migration and invasion by modulating signaling of EGFR. J. Clin. Endocrinol. Metab. 2014, 74, 5570.
- Marinkovich, M.P. Tumour microenvironment: Laminin 332 in squamous-cell carcinoma. Nat. Rev. Cancer 2007, 7, 370–380.





