Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gabriele Nasello | + 2763 word(s) | 2763 | 2021-05-17 11:14:29 | | | |
| 2 | Gabriele Nasello | Meta information modification | 2763 | 2021-05-18 17:25:09 | | | | |
| 3 | Vivi Li | Meta information modification | 2763 | 2021-05-19 03:24:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nasello, G.; Schiavi-Tritz, J. Hydrogel-Based Bone-On-Chips. Encyclopedia. Available online: https://encyclopedia.pub/entry/9800 (accessed on 07 February 2026).
Nasello G, Schiavi-Tritz J. Hydrogel-Based Bone-On-Chips. Encyclopedia. Available at: https://encyclopedia.pub/entry/9800. Accessed February 07, 2026.
Nasello, Gabriele, Jessica Schiavi-Tritz. "Hydrogel-Based Bone-On-Chips" Encyclopedia, https://encyclopedia.pub/entry/9800 (accessed February 07, 2026).
Nasello, G., & Schiavi-Tritz, J. (2021, May 18). Hydrogel-Based Bone-On-Chips. In Encyclopedia. https://encyclopedia.pub/entry/9800
Nasello, Gabriele and Jessica Schiavi-Tritz. "Hydrogel-Based Bone-On-Chips." Encyclopedia. Web. 18 May, 2021.
Copy Citation
The recent development of bone-on-chips (BOCs) holds the main advantage of requiring a low quantity of cells and material, compared to traditional In Vitro models. By incorporating hydrogels within BOCs, the culture system moved to a three dimensional culture environment for cells which is more representative of bone tissue matrix and function. The fundamental components of hydrogel-based BOCs, namely the cellular sources, the hydrogel and the culture chamber, have been tuned to mimic the hematopoietic niche in the bone aspirate marrow, cancer bone metastasis and osteo/chondrogenic differentiation.
bone-on-chip
bone microenvironment
bone hydrogels
tunable hydrogels
microfluidic bone treatments
bone models
1. Introduction
Musculoskeletal disorders and related bone diseases are one of the major causes of pain and disability as well as a social and economical burden for our society. Diseases such as osteoarthritis, bone fracture, osteoporosis, bone tumor and osteosarcopenia affect 1 of 2 adults in United States [1] and more than 100 million Europeans [2]. More specifically on bone alteration, the estimated incidence of bone fractures is 3 per 100 people per year in UK [3] and US [4]. Despite the intrinsic repair capacities of bone tissue, 5–10% of fractures are not self-healdling and described as non-unions and require expensive operative interventions [5]. Bone traumatic injuries cost $56 billion every year in US alone [4], while fractures associated to osteoporosis cost €37.5 billion in the largest European countries [6]. In search of reducing the societal and economical burden of bone diseases, experimental models of bone tissue are continuously evolving to recapitulate specific mechanisms of bone physiology, pathology or to evaluate the effect of potential therapies. Two types of models are available, ex vivo models where bone tissue are cultured outside of the body [7] and In Vitro models where cells are isolated and cultured in a 2D or 3D environment. In Vitro experimental bone models are more easily available than living explants and facilitate the culture of human cells in a controlled environment outside of living organisms [8]. Moreover, a high number of parameters can be tested with one single batch. Yet, traditional 2D In Vitro models are not suitable for long-term studies and may fail in recapitulating a clinically relevant environment due to the absence of all factors present in vivo [9], but their high output and outcome balance this weakness.
The lack of clinical relevance of 2D or the experimental challenge of large scale 3D In Vitro models, which includes the high number of cells necessary and necrosis in the core of large scaffold, led to the development of the organ-on-chip field. The organ-on-chip technologies have recently emerged from the synergy of microfabrication techniques and tissue engineering, aiming to replicate specific processes of organ functionality in sophisticated In Vitro microenvironments and reduce the amount of cells required. While the use of organ-on-chips for drug screening is steadily increasing [10], the combination of microengineered systems with primary patient cells is inspiring the field of regenerative medicine [11] as well as personalized medicine, since they facilitate the use of cells isolated from individual patients [12]. Different organ-on-chips were recently developed to predict the variability between individuals associated to specific biological processes, such as the permeability of the blood-brain barrier [13], the inflammation of the human airways [14], the proliferation of multiple myeloma cells [15] and the drug-induced hematotoxicity [16].
Compared to other tissues, Bone-On-Chip (BOC) platforms have mostly emerged in recent years. The first literature review on the development and challenges of BOC systems has been recently published, showing the main technical solutions adopted to study bone cell function, bone regeneration and its interaction with multiple tissues [17]. Starting from a monolayer of mouse osteocyte-like cell line to study mechano-regulation under oscillatory fluid flow [18], BOCs moved to three dimensional (3D) culture systems that investigated the osteocytic network formation [19][20] or the bone matrix mineralization process [21]. Indeed, 3D culture environments mimicking the extracellular matrix (ECM) provide representative systems of tissue function where hydrogels are an ideal candidate to reflect the matrix topography and properties.
2. Hydrogel-Based Bone-On-Chips
The development of organ-on-chips typically starts from a microfabrication technique, such as mask-based photolithography, etching precise microscale pattern into photosensitive materials, thus creating a mold [22]. Later, soft lithography replicates the master pattern in the microengineered device. For organ-on-chips applications, the soft elastomer poly(dimethylsiloxane) (PDMS) is the standard material used for the stamp, given its optical transparency, and biocompatibility. Moreover, PDMS can restore hydrophobicity after the stamp is bonded to a flat surface, which facilitates the filling of the culture chamber with hydrogel [23]. The final device consists of transparent polymeric microchannels where mechanical stimuli, such as laminar fluid flow, and biochemical gradients can be applied, while tissue-tissue interfaces can be replicated [24]. Any organ-on-chip requires one or multiple cell types and a microscale culture chamber. Hereafter, the culture chamber designs, the hydrogels and the cellular components used in BOCs are discussed.
2.1. Culture Chamber Designs
The complexity of culture chamber designs in BOCs is intrinsically related to the composition of the tissue. Indeed multi-cellular and multi-tissue interaction can be modelled within each device. In general, single channeldevices are preferred for cell migration studies, where cells can be tracked over time within the hydrogel while applying mechanical [25] or chemical stimuli [26][27][28]. Microposts between channels facilitate the identification of the region of interests while taking multiple images over time and support the hydrogel stability via surface tension [28][29]. Moreover, microposts separate culture chamber, thus confining cells and hydrogels in compartments where different culturing conditions can be applied [30].
The definition of compartments in organ-on-chips is one of the main advantage of microengineered technologies over traditional culture techniques. By means of computer aided design (CAD), it is possible to tune the geometry of each single channel and the way multiple channels communicate with each other [31]. An example of effective culture chamber design in BOC is the one proposed by Mei et al., who studied cancer cell extravasation in bone tissue by creating channels for osteocytes and a lumen. In the osteocyte channel, cells could be selectively stimulated with oscillatory fluid flow, while the lumen channel consisted of a cylindrical hydrogel structure coated with endothelial cells and seeded with breast cancer cells. Moreover, the distance between the osteocyte and the lumen channel was 300 µm, to recapitulate the physiological distance between osteocytes and effectors cells [32]. Similarly, a bone-marrow-on-chip was recently developed based on two channels separated by a porous membrane. The two channels consisted of a hematopoietic and a vascular compartment. The in vivo functionality of the vascular lumen was replicated by feeding the whole chip exclusively via perfusion through the vascular compartment that was covered with endothelial cells [16]. For the microfabrication details of organ-on-chip devices comprising multiple cell types embedded in hydrogels, we recommend reading the protocol developed by Shin et al. [23], Huh et al. [33] and Novak et al. [34].
Besides using channels as culture chambers, microengineered system can induce chemical gradients within the culture environment. In addition, microfluidic devices facilitate the application of independent fluid flows for different channels. Thus, a system of two serpentine channels perfused by different cell culture media induced an osteo/chondrogenic gradient within a single hydrogel. In this way, the same hydrogel-based environment induced a differentiation gradient for MSCs mimicking the interface between bone and cartilage [35].
2.2. Hydrogels
Given their remarkable biocompatibility and non-toxicity, natural hydrogels are often selected to imitate the native ECM in BOC systems (Table 1). The hydrogel choice depends on the specific environment to be modeled. For example, the formation of a fibrin matrix immediately after a bone injury makes fibrin gels a suitable model to study the healing of bone. By embedding osteoprogenitor cells, a fibrin gel can generate a healing bone-mimicking (BMi) microenvironment and induced the functional formation of a microvascular network [36]. Moreover, in the same project, the same microenvironment recreates a bone inflammatory model with the addition of a macrophage-like cell line (RAW264.7 cells).
Table 1. Selection of recent studies using hydrogel-based bone-on-chips to model physiological or pathological bone microenvironments. Hydrogel concentrations are between brackets and expressed in mg/mL or weight/volume%. Abbreviations: hBM human bone marrow derived, MSC mesenchymal stem cell, MDA-MB-231 human mammary adenocarcinoma cell (high invasion capacity), HUVEC human umbilical vein endothelial cell, MLO-Y4 murine osteocyte-like cell Line, OD osteoblast-differentiated, BMSC bone marrow stromal cell, SUP-B15 acute lymphoblastic leukemia cell line, HOB primary human osteoblasts, HS5 human bone marrow stromal cell line, DBP bone-inducing demineralized bone powder, BMP bone morphogenetic protein, HA hydroxyapatite, SW620 human colon cancer, MKN74 human gastric cancer, LF human lung fibroblast, ADMSC adipose-derived mesenchymal stem cell, ECM extracellular matrix.
| Goal | Cells | Chamber | Hydrogel | Main findings | Ref |
|---|---|---|---|---|---|
| Cancer metastasis | MDA-MB-231 hBM-MSC HUVEC | 4 independent gel chambers | Osteo-cell conditioned collagen (6.0) | Extravasation of breast cancer cells is influenced by cell receptor CXCR2 | [37] |
| MDA-MB-231 MLO-Y4 HUVEC | Lumen channel and osteocyte channel | Mix of collagen I (5.5) and Matrigel (2.5) | Mechanically stimulated osteocytes reduced breast cancer cell migration | [32] | |
| hBM-MSC OD-hBM-MSC HUVEC MDA-MB-231 | 1 gel channel | Fibrin (5.0) | Bone-mimicking microenvironment induced the highest breast cancer extravasation rates | [36] | |
| OD-MSC HUVEC abc LF abcde MDA-MB-231 | 1 channel and endothelial monolayer on lateral sides | Mix of fibrin (2.5) and collagen I (0.2) | 3D vascularized spheroids-on-chip enhances breast cancer cell migration | [38] | |
| Cancer cell (SW620 or MKN74) HUVEC abc LF | Separate channels for tumor micro- environment and fibroblast co-culture | HA (0.2–0.4%)/ fibrin (2.5) composite | Inhibitory effect of HA in colon cancer cell migration and angiogenic sprout formation | [39] | |
| Osteochondro- genesis | ADMSC | 2 lateral serpentine channels and 1 linear middle channel | Agarose (2.5%) | Simultaneous osteogenic and chondrogenic differentiation of stem cells | [35] |
| Osteogenic differentiation | HOB | 3 channels separated by trapezoidal posts | Collagen I (6.0) | Recapitulation of osteoblast maturation toward osteocytes and matrix mineralization | [29] |
| Bone marrow physiology | Subcutaneous implantation prior to In Vitro culture | Cylindrical chamber for the explant and perfusion channels | Collagen I (3.0) with DBP, BMP2, BMP4 | Reconstitution of hematopoietic niche physiology and response to radiation toxicity | [40] |
| Bone marrow pathology | CD34+ cell BMSC HUVEC | Apical and basal channels separated by membrane | Mix of fibrin (5.0) and collagen I (0.2) | Prediction of clinically observed haemato- toxicities and representation of Shwachman–Diamond syndrome | [16] |
| SUP-B15 BMSC HOB | 4 channels | Collagen I (2.0 and 4.0) | Bone marrow microenvironment enhances leukemia cell survival during drug treatment | [41] | |
| leukemic cell (U937, HL60 or K562) HUVEC HS5 | Gel channel, leukemic channel and endothelial channel | Collagen I (2.5 and 5.0) | Angiogenesis in bone marrow promotes leukemia cell proliferation and survival | [42] | |
| Vascularized bone tissue | HUVEC LF | 4 parallel channels separated by microposts | HA (0.05–0.5%)/ fibrin (-) composite | HA enhances sprouting speed and increased lumen size, sprout length and number | [43] |
| Cell migration | HOB | 1 channel | Collagen I (4.0) | Interstitial fluid flow alters hydrogel microstructure and cell migration | [25] |
| HOB | 1 channel | Collagen I (4.0) | The inhibition of ECM degradation reduces cell motility | [26] | |
| hBM-MSC | 1 channel | Mix (1:1) of collagen (2.0) and Matrigel | Dose-dependent chemoattractant ability of different chemokines | [28] |
In search of a model representing mature bone, fibrin was mixed with type I collagen which increased the hydrogel stiffness and its mechanical stability [44]. Due to its ubiquitous presence in the bone matrix, hydrogel-based BOC systems normally used type I collagen when culturing primary Human Osteoblasts (HOBs) (Table 1). The collagen mechanical properties varied between the different applications of the BOCs. In general, collagen concentration was higher when the system meant to induces osteogenic differentiation and mineralization [29][37], while it was lower when studying osteoblast migration [25][26]. Collagen-based hydrogels have a fibrous architecture whose interaction with the embedded cells alters both the hydrogel mechanical properties and the cellular activity within the BOC. It has been demonstrated that collagen fiber reorientation due to interstitial fluid flow increases osteoblasts migration [25]. Moreover, osteoblasts remodel the collagen hydrogel with proteolytic enzymes, the metalloproteinases, which in turn regulate cell migration through the matrix [26].
Hydrogels mechanical properties can be enhanced with composite systems. Besides mixing with fibrin, collagen I was also mixed with Matrigel prior to load within BOC devices (Table 1). Given that Matrigel is a gelatinous protein mixture, a composite hydrogel made of collagen I and Matrigel provides a variety of structural proteins and growth factors resembling the native ECM [45]. Although the incorporation of a bioactive inorganic phase in hydrogels has been widely investigated to boost hard tissue regeneration [46], bone formation was never assessed in hydrogel-based BOC while incorporating calcium phosphates or bioglasses. However, HA nanoparticles were incorporated in fibrin hydrogels and loaded into BOC systems and showed the inhibitory effect HA in cancer cell migration [39] and a stimulative effect on endothelial sprouting [43]. Instead of incorporating inorganic phases, recent hydrogel-based BOCs recreated mineralized microenvironments by combining a collagen I hydrogel with osteogenic factors [40] or by culturing osteo-differentiated MSCs that deposited newly mineralized matrix [37].
2.3. Cell Interactions
The selection and the number of different cell types to introduce in a BOC system includes, but it is not limited to, cells normally residing in the bone tissue, while the interaction with other tissues requires the use of different cell sources. The cellular component of the bone matrix is usually represented by osteoblasts or osteocyteswhile MSC and osteoclast precursors are included to study bone remodelling or to create a bone marrow niche. MSCs are routinely obtained from bone marrow or adipose tissue, and used as osteoprogenitor cells in organ-on-chip mimicked the mineralizing microenvironment [36][38] or osteochondral bone formation [35]. On the other hand, MSCs were maintained In Vitro to recapitulate the bone marrow microenvironment. Indeed, once seeded in a bone-marrow-on-chip with CD34+ cells (hematopoietic stem cells), it has supported white and red blood cells differentiation over 4 weeks of culture while improving the maintenance of CD34+ progenitors over traditional culture methods [16] (Figure 1A). A different approach from the traditional isolation and seeding of primary human cells has been proposed to recapitulate the physiology of bone marrow. Briefly, it consisted of a prior in vivo implantation of an hydrogel-based bone microenvironment. After 8 weeks, the hydrogel was populated by multiple hematopoietic cells and it was cultured in a microfluidic device after explantation to recapitulate a hematopoietic niche In Vitro. Bone-marrow-on-chips and whole marrow from live mice showed comparable resistance to radiation, unlike stroma-supported cultures on a dish [40].
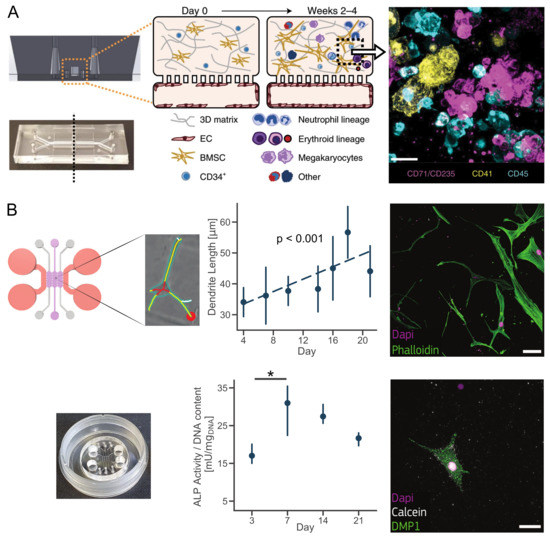
Figure 1. Inducing cell differentiation in hydrogel-based bone-on-chips. (A) A vascularized human bone marrow-on-chip was developed with optically clear poly(dimethylsiloxane) channels. In the top channel, hematopoietic stem cells (CD34+) were seeded, while endothelial cells (EC) created a vascular lumen in the bottom channel. After 2 weeks of In Vitro culture, hematopoietic stem cells differentiated in multiple blood cell types (magenta: erythroid lineage; yellow: megakaryocyte lineage; blue: neutrophil and other haematopoietic lineages). Scale bar, 20 µm. Image adapted from [16]. (B) Collagen-based bone-on-chip modeling primary human osteoblast differentiation into osteocytes. Cells increased primary protrusion length over time and exhibited dendritic morphology after 21 days of culture. Osteogenic differentiation was confirmed by an increasein alkaline phosphatase activity and synthesis of Dental Matrix Protein 1 (DMP1). * represents statistical significance (p < 0.05). Scale bar, 50 µm. Image adapted from [29]. Images were taken with permission from publishers.
In view of modeling the last stages of osteogenesis, HOB can be isolated from trabecular or cortical bone tissue. It has been showed that HOBs cultured in BOCs experience the specific changes in cellular morphology, protein secretion and proliferation observed in vivo [29] (Figure 1B). Due to the technical complexity of isolating primary human osteocytes from the mineralized bone, the murine osteocyte cell line MLO-Y4 was recently used in microfluidic systems to investigate the mechano-regulatory action of osteocytes [32]. Results showed, for the first time in a microfluidic device, that mechanically stimulated osteocytes reduced breast cancer extravasation [32]. The use of primary human osteocytes, or fully differentiated HOBs, in hydrogel-based BOCs will be critical for future patient-specific applications.
Besides including different cell types involved in the development, growth and remodeling of bones, a more realistic bone microenvironment requires modeling interactions between tissues made of those cells. For example, vascular tissue interacts with bone forming cells and affects both bone pathology and physiology. Indeed one BOC devicewas designed to deposit a layer of Human Umbilical Vein Endothelial Cells (HUVECs) on the external side of the hydrogelto recreate the interface between blood vessels and the bone matrix, and to simulate the extravasation process of cancer cells [37][42] (Figure 2). Indeed, the use of organ-on-chips facilitates the interaction between three to four different cells types to model the interaction between tumors, vascular and bone tissue. A few studies include fibroblasts when mimicking a tumor microenvironement since stromal cells are expected to nurture the tumor microenvironment and influence metastasis of the cancer [39][38]. Altogether those microengineered devices with endothelial cells contributed to identify the molecular pathways involved in the extravasation of breast cancer cells in bone [37], showed that the bone marrow microenvironment facilitates leukemia cell survival during drug treatment [41], and generated a vascularized network inside cell spheroids that permit to transport nutrients and cells to study metastasis [38].
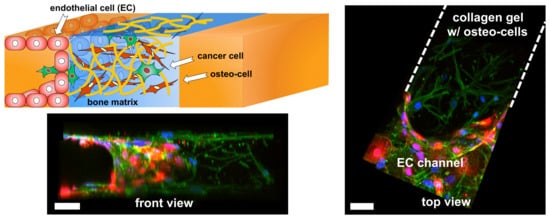
Figure 2. On-chip device modeling breast cancer metastasis in the bone tissue. Microfluidic device modeling breast cancer cell extravasation towards the bone tissue. Tri-culture system where osteo-differentiated mesenchymal stem cells created a bone-like environment by conditioning a collagen hydrogel. After seeding a monolayer of endothelial cells on the edge of the collagen hydrogel, breast cancer cells were introduced and their extravasation ability was assessed. Scale bar, 50 µm. Image adapted from [37]. Images were taken with permission from publishers.
References
- United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States (BMUS), 3rd ed; United States Bone and Joint Initiative: Rosemont, IL, USA, 2014; p. 711. Available online: (accessed on 1 March 2021).
- Plannel, J.; Navarro, M. Challenges of bone repair. In Bone Repair Biomaterials; Elsevier: Amsterdam, The Netherlands, 2009; pp. 3–24.
- Donaldson, L.J.; Reckless, I.P.; Scholes, S.; Mindell, J.S.; Shelton, N.J. The epidemiology of fractures in England. J. Epidemiol. Community Health 2008, 62, 174–180.
- Buza, J. Bone healing in 2016. Clin. Cases Miner. Bone Metab. 2016.
- Einhorn, T.A. Enhancement of fracture-healing. J. Bone Jt. Surg. 1995, 77, 940–956.
- Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility fractures in Europe: Burden, management and opportunities. Arch. Osteoporos. 2020, 15, 59.
- Cramer, E.E.A.; Ito, K.; Hofmann, S. Ex vivo Bone Models and Their Potential in Preclinical Evaluation. Curr. Osteoporos. Rep. 2021, 19, 75–87.
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5.
- Boussommier-Calleja, A.; Li, R.; Chen, M.B.; Wong, S.C.; Kamm, R.D. Microfluidics: A New Tool for Modeling Cancer–Immune Interactions. Trends Cancer 2016, 2, 6–19.
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278.
- Harink, B.; Le Gac, S.; Truckenmüller, R.; van Blitterswijk, C.; Habibovic, P. Regeneration-on-a-chip? The perspectives on use of microfluidics in regenerative medicine. Lab A Chip 2013, 13, 3512.
- Van den Berg, A.; Mummery, C.L.; Passier, R.; van der Meer, A.D. Personalised organs-on-chips: Functional testing for precision medicine. Lab A Chip 2019, 19, 198–205.
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell 2019, 24, 995–1005.e6.
- Benam, K.H.; Villenave, R.; Lucchesi, C.; Varone, A.; Hubeau, C.; Lee, H.H.; Alves, S.E.; Salmon, M.; Ferrante, T.C.; Weaver, J.C.; et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses In Vitro. Nat. Methods 2016, 13, 151–157.
- Zhang, W.; Lee, W.Y.; Siegel, D.S.; Tolias, P.; Zilberberg, J. Patient-Specific 3D Microfluidic Tissue Model for Multiple Myeloma. Tissue Eng. Part C Methods 2014, 20, 663–670.
- Chou, D.B.; Frismantas, V.; Milton, Y.; David, R.; Pop-Damkov, P.; Ferguson, D.; MacDonald, A.; Vargel Bölükbaşı, Ö.; Joyce, C.E.; Moreira Teixeira, L.S.; et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat. Biomed. Eng. 2020, 4, 394–406.
- Mansoorifar, A.; Gordon, R.; Bergan, R.C.; Bertassoni, L.E. Bone-on-a-Chip: Microfluidic Technologies and Microphysiologic Models of Bone Tissue. Adv. Funct. Mater. 2020, 2006796.
- You, L.; Temiyasathit, S.; Lee, P.; Kim, C.H.; Tummala, P.; Yao, W.; Kingery, W.; Malone, A.M.; Kwon, R.Y.; Jacobs, C.R. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone 2008, 42, 172–179.
- Sun, Q.; Choudhary, S.; Mannion, C.; Kissin, Y.; Zilberberg, J.; Lee, W.Y. Ex vivo construction of human primary 3D—Networked osteocytes. Bone 2017, 105, 245–252.
- Leclerc, E.; David, B.; Griscom, L.; Lepioufle, B.; Fujii, T.; Layrolle, P.; Legallaisa, C. Study of osteoblastic cells in a microfluidic environment. Biomaterials 2006, 27, 586–595.
- Hao, S.; Ha, L.; Cheng, G.; Wan, Y.; Xia, Y.; Sosnoski, D.M.; Mastro, A.M.; Zheng, S.Y. A Spontaneous 3D Bone-On-a-Chip for Bone Metastasis Study of Breast Cancer Cells. Small 2018, 14, 1–10.
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754.
- Shin, Y.; Han, S.; Jeon, J.S.; Yamamoto, K.; Zervantonakis, I.K.; Sudo, R.; Kamm, R.D.; Chung, S. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat. Protoc. 2012, 7, 1247–1259.
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260.
- Del Amo, C.; Olivares, V.; Cóndor, M.; Blanco, A.; Santolaria, J.; Asín, J.; Borau, C.; García-Aznar, J.M. Matrix architecture plays a pivotal role in 3D osteoblast migration: The effect of interstitial fluid flow. J. Mech. Behav. Biomed. Mater. 2018, 83, 52–62.
- Movilla, N.; Borau, C.; Valero, C.; García-Aznar, J. Degradation of extracellular matrix regulates osteoblast migration: A microfluidic-based study. Bone 2018, 107, 10–17.
- Moreno-Arotzena, O.; Mendoza, G.; Cóndor, M.; Rüberg, T.; García-Aznar, J.M. Inducing chemotactic and haptotactic cues in microfluidic devices for three-dimensional In Vitro assays. Biomicrofluidics 2014, 8, 064122.
- Yoon, D.; Kim, H.; Lee, E.; Park, M.H.; Chung, S.; Jeon, H.; Ahn, C.H.; Lee, K. Study on chemotaxis and chemokinesis of bone marrow-derived mesenchymal stem cells in hydrogel-based 3D microfluidic devices. Biomater. Res. 2016, 20, 25.
- Nasello, G.; Alamán-Díez, P.; Schiavi, J.; Pérez, M.Á.; McNamara, L.; García-Aznar, J.M. Primary Human Osteoblasts Cultured in a 3D Microenvironment Create a Unique Representative Model of Their Differentiation Into Osteocytes. Front. Bioeng. Biotechnol. 2020, 8.
- Moraes, C.; Mehta, G.; Lesher-Perez, S.C.; Takayama, S. Organs-On-a-Chip: A Focus on Compartmentalized Microdevices. Ann. Biomed. Eng. 2012, 40, 1211–1227.
- Cóndor, M.; Rüberg, T.; Borau, C.; Piles, J.; García-Aznar, J. A web-based application for automated quantification of chemical gradients induced in microfluidic devices. Comput. Biol. Med. 2018, 95, 118–128.
- Mei, X.; Middleton, K.; Shim, D.; Wan, Q.; Xu, L.; Ma, Y.H.V.; Devadas, D.; Walji, N.; Wang, L.; Young, E.W.K.; et al. Microfluidic platform for studying osteocyte mechanoregulation of breast cancer bone metastasis. Integr. Biol. 2019, 11, 119–129.
- Huh, D.; Kim, H.J.; Fraser, J.P.; Shea, D.E.; Khan, M.; Bahinski, A.; Hamilton, G.A.; Ingber, D.E. Microfabrication of human organs-on-chips. Nat. Protoc. 2013, 8, 2135–2157.
- Novak, R.; Didier, M.; Calamari, E.; Ng, C.F.; Choe, Y.; Clauson, S.L.; Nestor, B.A.; Puerta, J.; Fleming, R.; Firoozinezhad, S.J.; et al. Scalable Fabrication of Stretchable, Dual Channel, Microfluidic Organ Chips. J. Vis. Exp. 2018.
- Shi, X.; Zhou, J.; Zhao, Y.; Li, L.; Wu, H. Gradient-Regulated Hydrogel for Interface Tissue Engineering: Steering Simultaneous Osteo/Chondrogenesis of Stem Cells on a Chip. Adv. Healthc. Mater. 2013, 2, 846–853.
- Jeon, J.S.; Bersini, S.; Gilardi, M.; Dubini, G.; Charest, J.L.; Moretti, M.; Kamm, R.D. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc. Natl. Acad. Sci. USA 2015, 112, 214–219.
- Bersini, S.; Jeon, J.S.; Dubini, G.; Arrigoni, C.; Chung, S.; Charest, J.L.; Moretti, M.; Kamm, R.D. A microfluidic 3D In Vitro model for specificity of breast cancer metastasis to bone. Biomaterials 2014, 35, 2454–2461.
- Sano, E.; Mori, C.; Nashimoto, Y.; Yokokawa, R.; Kotera, H.; Torisawa, Y.S. Engineering of vascularized 3D cell constructs to model cellular interactions through a vascular network. Biomicrofluidics 2018, 12, 042204.
- Ahn, J.; Lim, J.; Jusoh, N.; Lee, J.; Park, T.E.; Kim, Y.; Kim, J.; Jeon, N.L. 3D Microfluidic Bone Tumor Microenvironment Comprised of Hydroxyapatite/Fibrin Composite. Front. Bioeng. Biotechnol. 2019, 7.
- Torisawa, Y.S.; Spina, C.S.; Mammoto, T.; Mammoto, A.; Weaver, J.C.; Tat, T.; Collins, J.J.; Ingber, D.E. Bone marrow-on-a-chip replicates hematopoietic niche physiology In Vitro. Nat. Methods 2014, 11, 663–669.
- Bruce, A.; Evans, R.; Mezan, R.; Shi, L.; Moses, B.S.; Martin, K.H.; Gibson, L.F.; Yang, Y. Three-Dimensional Microfluidic Tri-Culture Model of the Bone Marrow Microenvironment for Study of Acute Lymphoblastic Leukemia. PLoS ONE 2015, 10, e0140506.
- Zheng, Y.; Sun, Y.; Yu, X.; Shao, Y.; Zhang, P.; Dai, G.; Fu, J. Angiogenesis in Liquid Tumors: An In Vitro Assay for Leukemic-Cell-Induced Bone Marrow Angiogenesis. Adv. Healthc. Mater. 2016, 5, 1014–1024.
- Jusoh, N.; Oh, S.; Kim, S.; Kim, J.; Jeon, N.L. Microfluidic vascularized bone tissue model with hydroxyapatite-incorporated extracellular matrix. Lab A Chip 2015, 15, 3984–3988.
- Rao, R.R.; Peterson, A.W.; Ceccarelli, J.; Putnam, A.J.; Stegemann, J.P. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis 2012, 15, 253–264.
- Zhao, Z.; Vizetto-Duarte, C.; Moay, Z.K.; Setyawati, M.I.; Rakshit, M.; Kathawala, M.H.; Ng, K.W. Composite Hydrogels in Three-Dimensional In Vitro Models. Front. Bioeng. Biotechnol. 2020, 8.
- Gkioni, K.; Leeuwenburgh, S.C.; Douglas, T.E.; Mikos, A.G.; Jansen, J.A. Mineralization of Hydrogels for Bone Regeneration. Tissue Eng. Part B Rev. 2010, 16, 577–585.
More
Information
Subjects:
Engineering, Biomedical; Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
3 times
(View History)
Update Date:
19 May 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




